Introduction
Recent research has shed light on the crucial factors affecting myoblast regeneration, a key process in muscle repair. Myoblasts, the precursor cells to muscle fibers, are subjected to mechanical forces such as shear stress during muscle regeneration. This study aimed to explore how these mechanical forces, combined with changes in the extracellular matrix (ECM) stiffness associated with aging, influence myoblast proliferation and differentiation.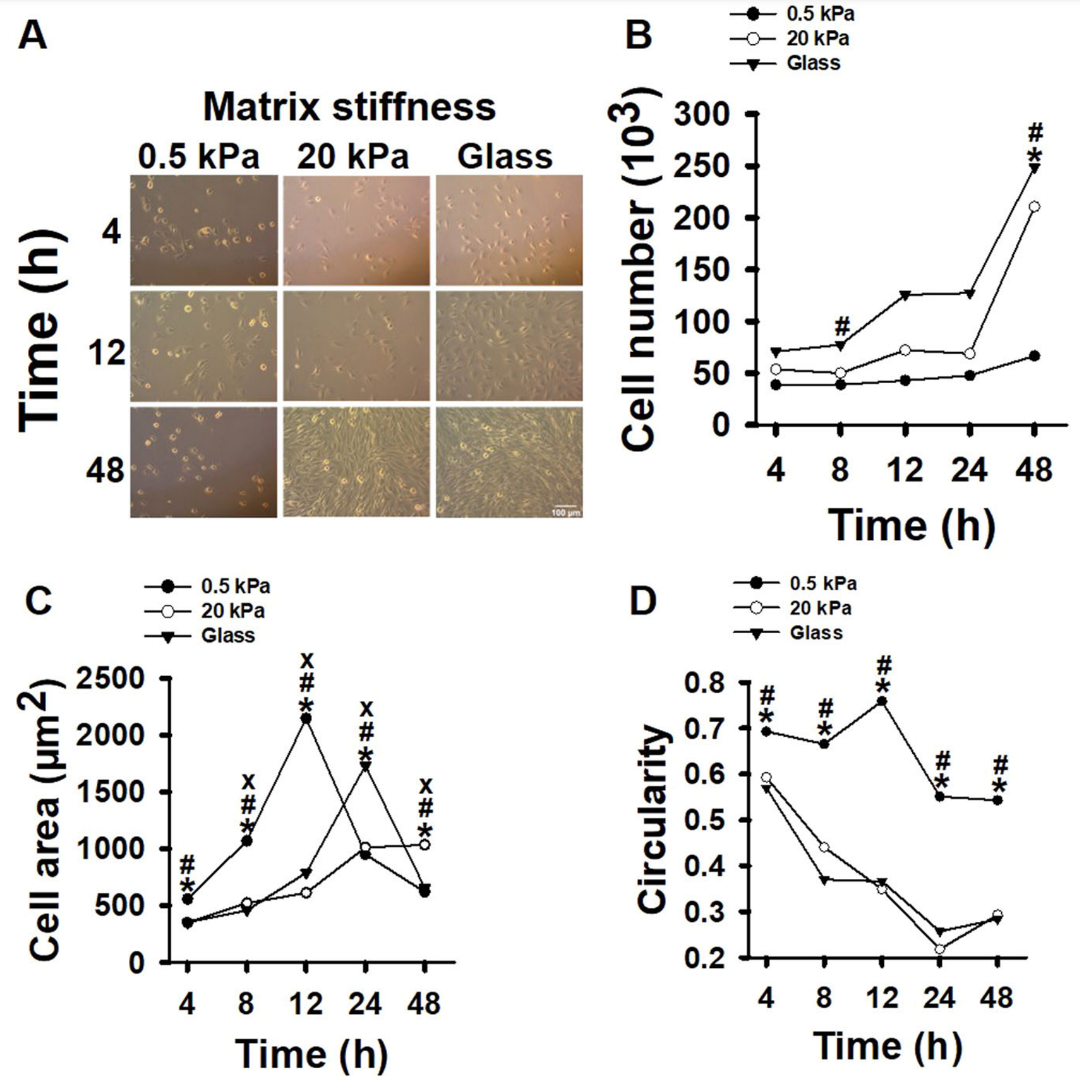
Study Overview
The study investigated two main questions:
- How do matrix stiffness and pulsating fluid shear stress affect myoblast proliferation and the expression of differentiation-associated genes?
- Does matrix stiffness alter the mechanoresponse of myoblasts to pulsating fluid shear stress?
To answer these, researchers cultured myoblasts on polyacrylamide gel matrices with varying stiffness levels, representing young (“soft,” 0.5 kPa) and aged (“stiff,” 20 kPa) ECM, as well as on very stiff glass matrices (40 MPa). The cells were then subjected to pulsating fluid shear stress (3 Pa/s or 4 Pa/s at 1 Hz) for one hour.
Key Findings
The findings revealed that matrix stiffness significantly impacts myoblast behavior:
- Proliferation: Myoblasts showed increased proliferation on stiff matrices compared to soft ones.
- Differentiation: The ability of myoblasts to differentiate was reduced on stiff matrices.
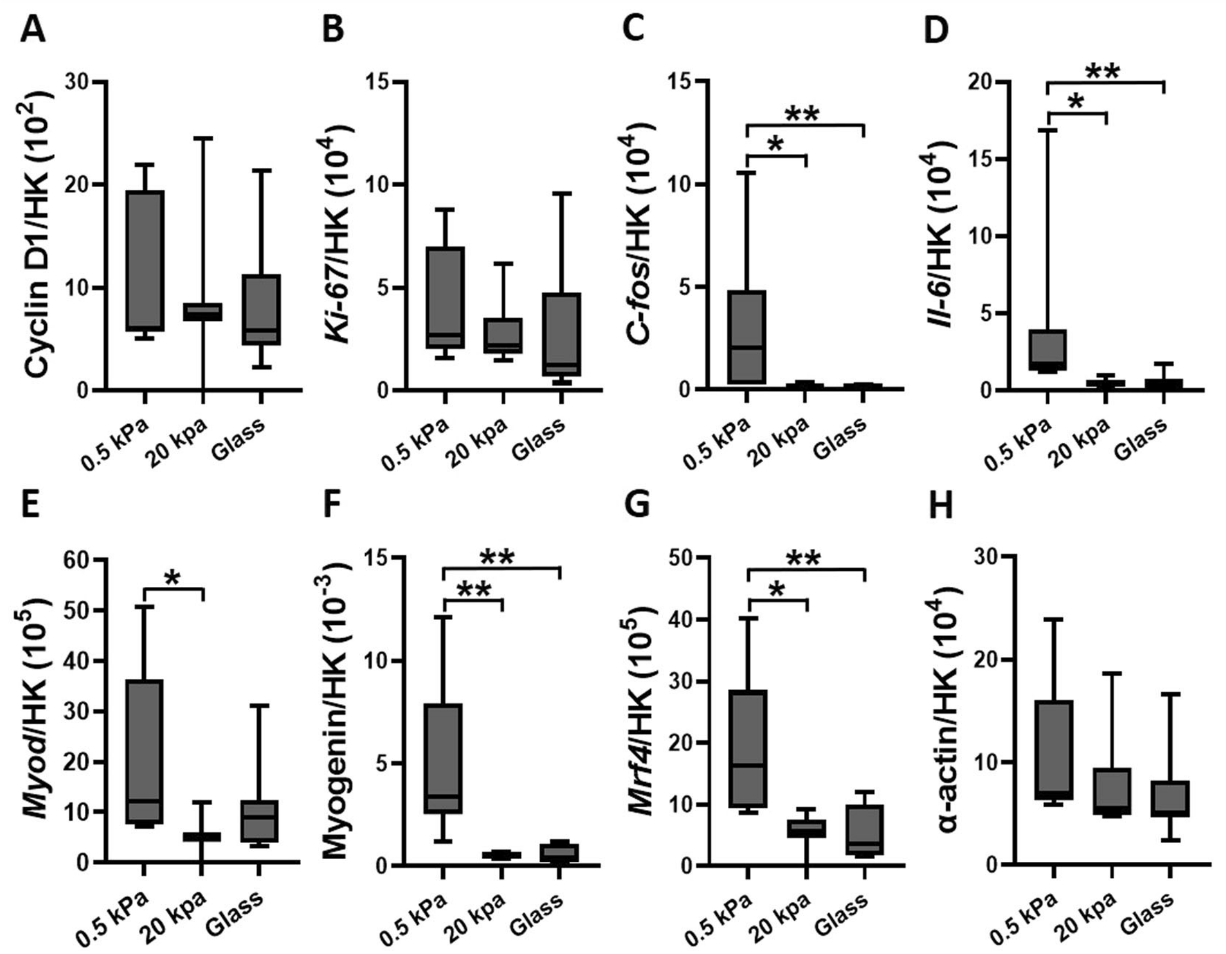
When exposed to pulsating fluid shear stress, the gene expression responses were also influenced by matrix stiffness:
- On stiff matrices, shear stress upregulated genes associated with proliferation (C-fos and Il-6) and cytoskeletal α-actin.
- On soft matrices, shear stress downregulated genes associated with cytoskeletal α-actin and differentiation (Myogenin).
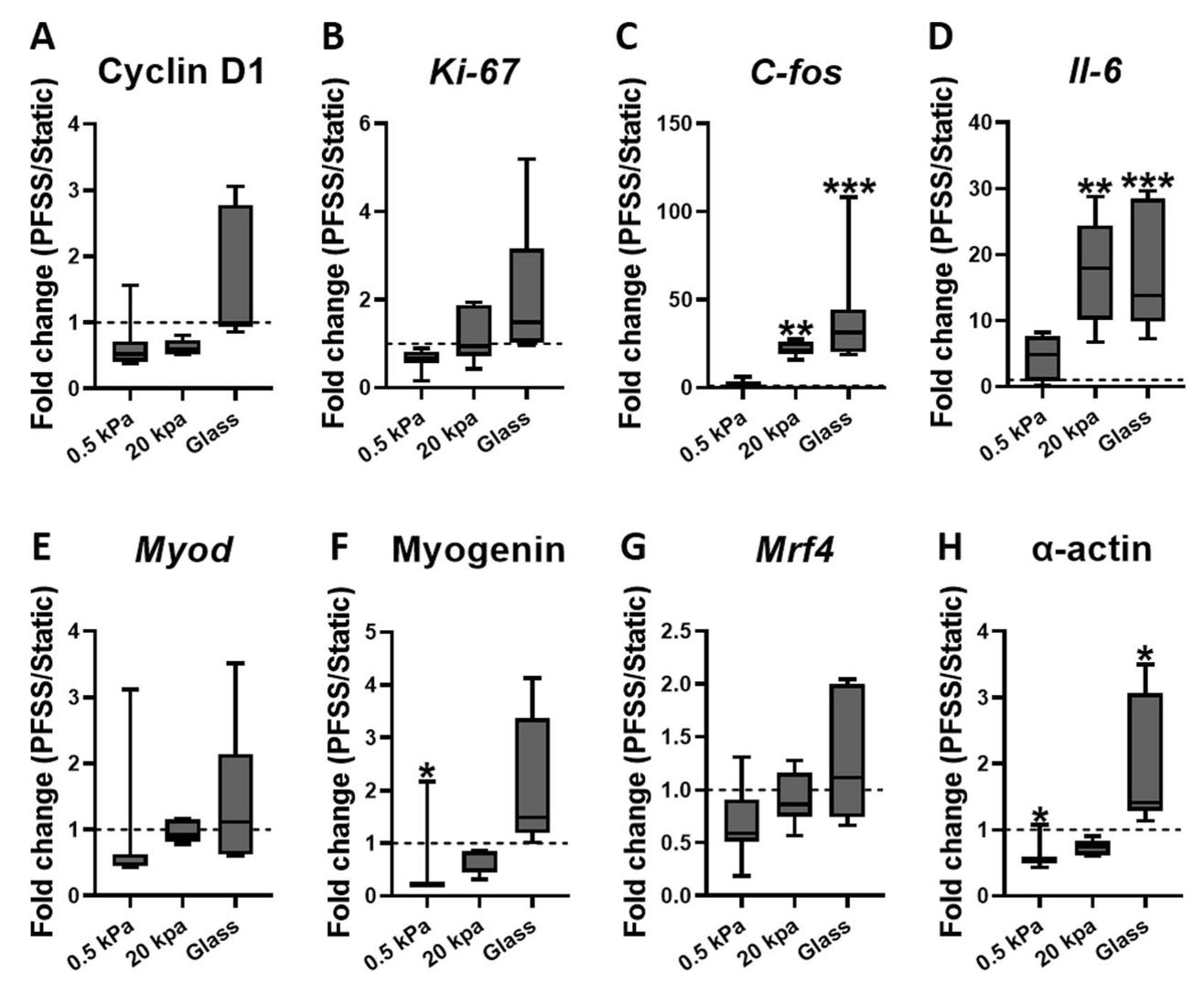
Conclusion
This study concludes that both ECM stiffness and fluid shear stress are critical in regulating myoblast proliferation and differentiation. Stiffer matrices, akin to those found in aged muscles, promote myoblast proliferation but hinder their differentiation into new muscle fibers. This implies that increased ECM stiffness due to aging or disease could impair muscle regeneration.
Moreover, shear stress appears essential for coordinating myofiber regeneration, with ECM stiffness modulating this response. The results suggest that muscle stretching and contraction, which generate shear stress, are beneficial for muscle regeneration. However, in aging muscles, the strain magnitude and shear load might need adjustment to maintain effective regeneration due to the altered stiffness of the ECM.
Implications
These findings highlight the importance of considering ECM stiffness and mechanical forces in strategies aimed at improving muscle regeneration, especially in the context of aging and muscle-related diseases. Future research and therapeutic approaches could focus on optimizing mechanical environments to enhance myoblast differentiation and muscle repair.
Read The Full Article Here.
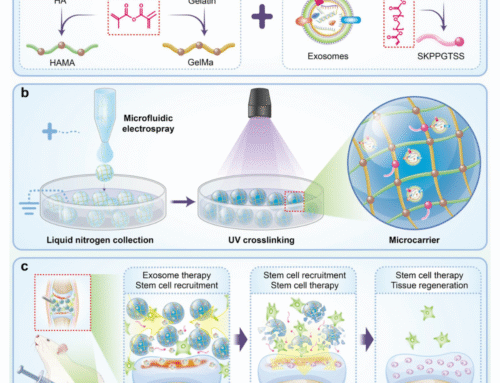
At Smart MCs, we offer customised microcarriers and hydrogels with tunable stiffness to enhance cell proliferation and differentiation for a range of applications. Get in touch with our experts today to explore your hydrogel and microcarrier needs!



Leave A Comment