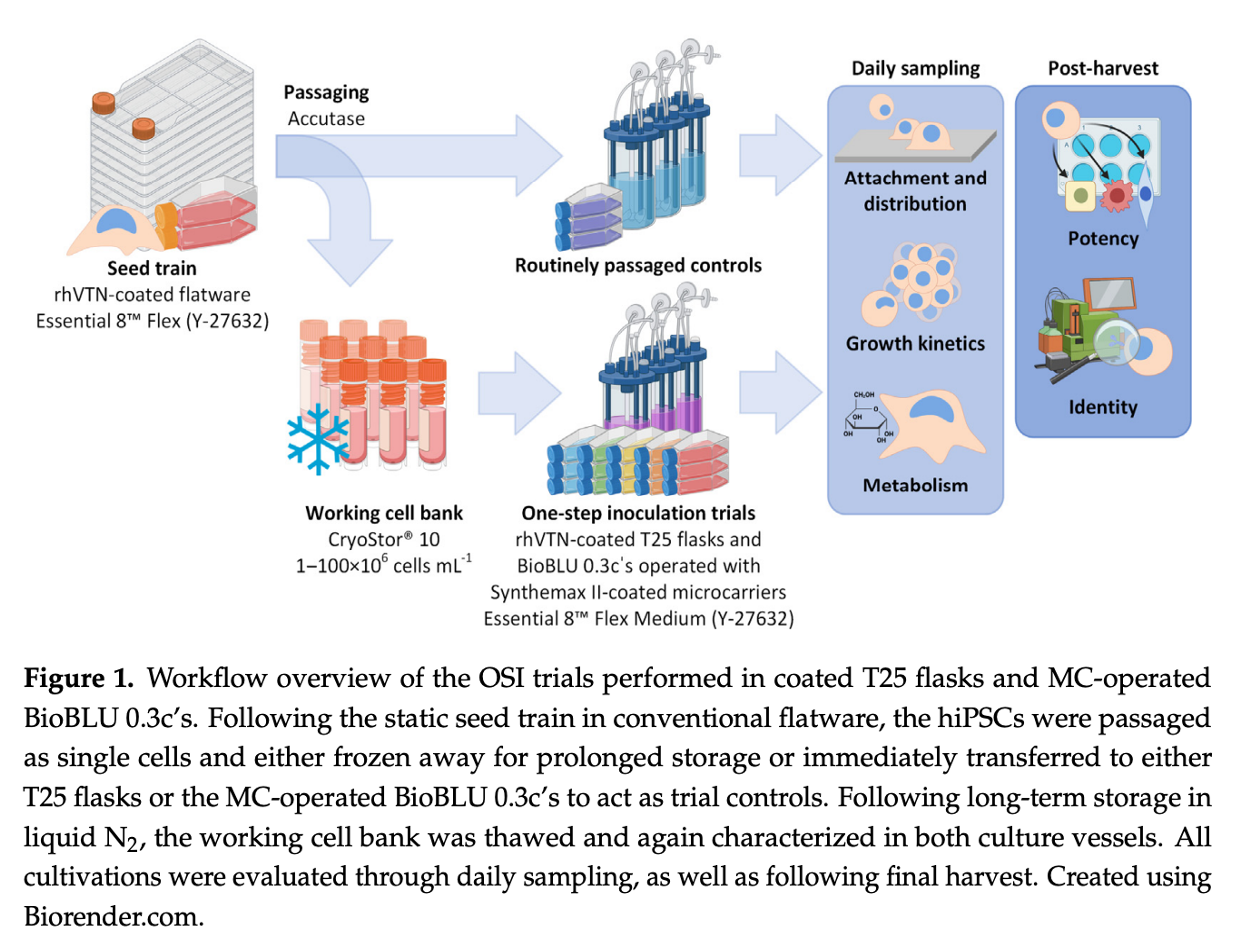
A recent study has shed light on a novel process intensification strategy for the expansion of human induced pluripotent stem cells (hiPSCs), which holds promise for enhancing the manufacturing of hiPSC-based therapeutics. The research focused on evaluating a one-step inoculation (OSI) approach, commonly used in antibody and vaccine production, and its potential application to hiPSC expansion in both static flasks and microcarrier-operated stirred bioreactors.
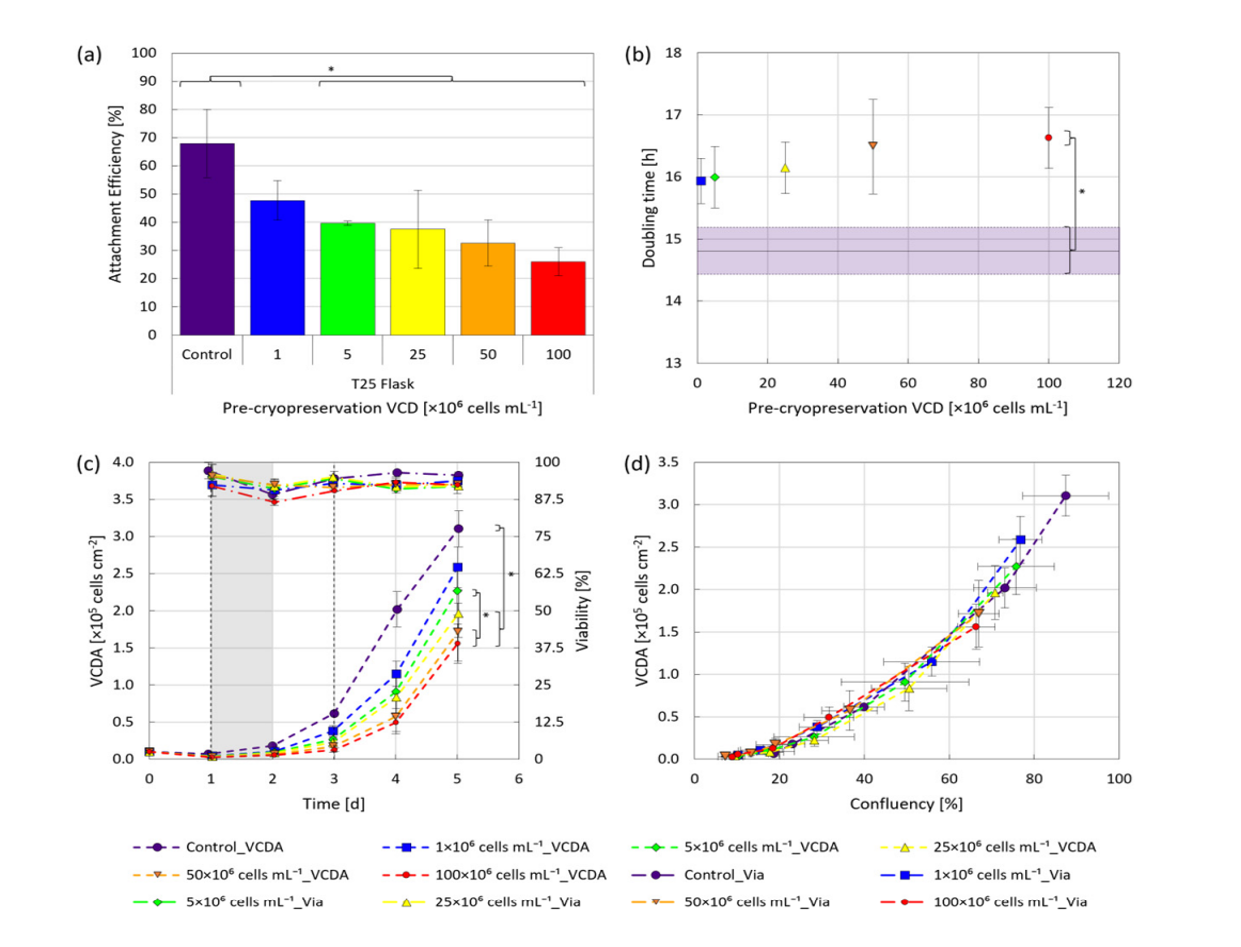
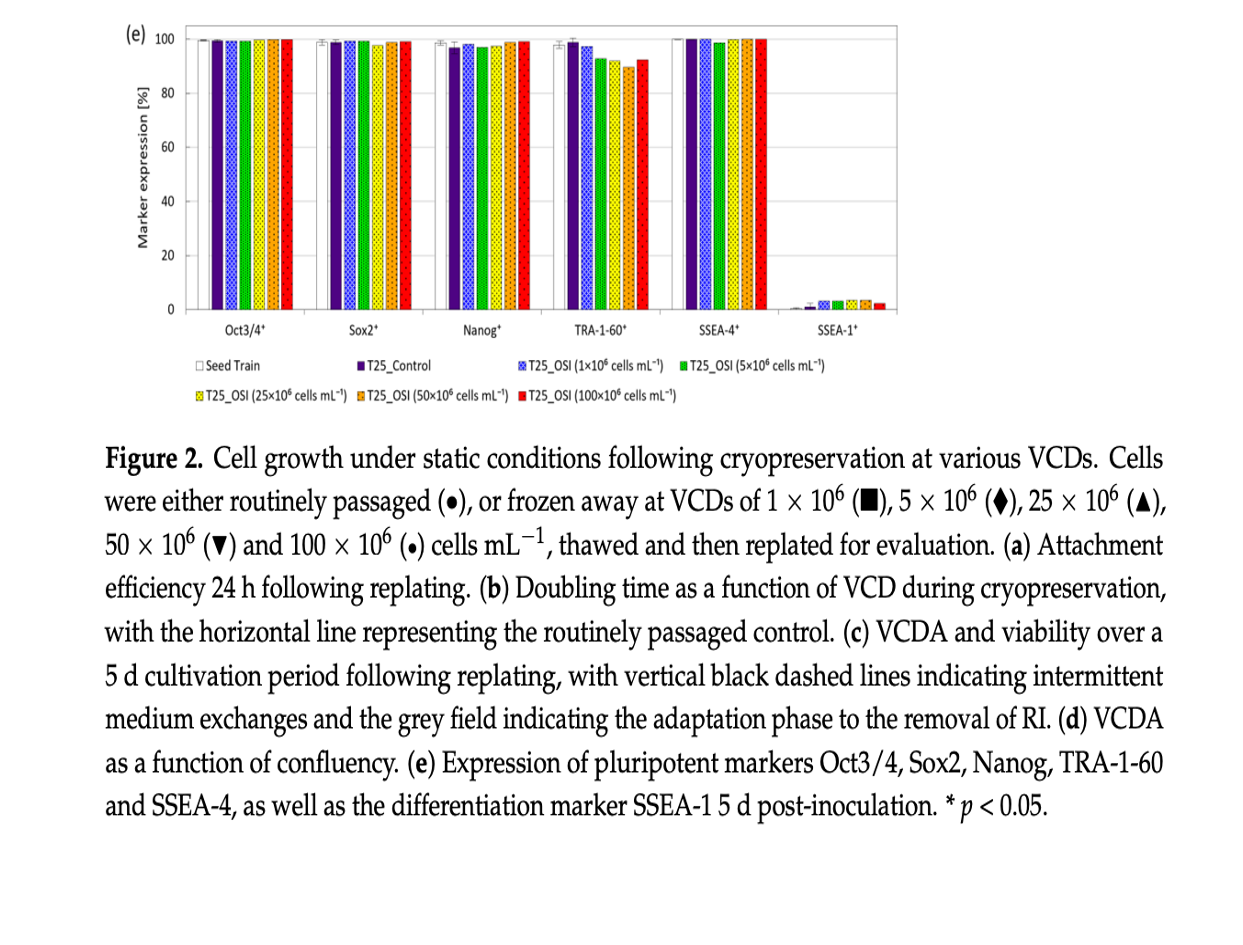
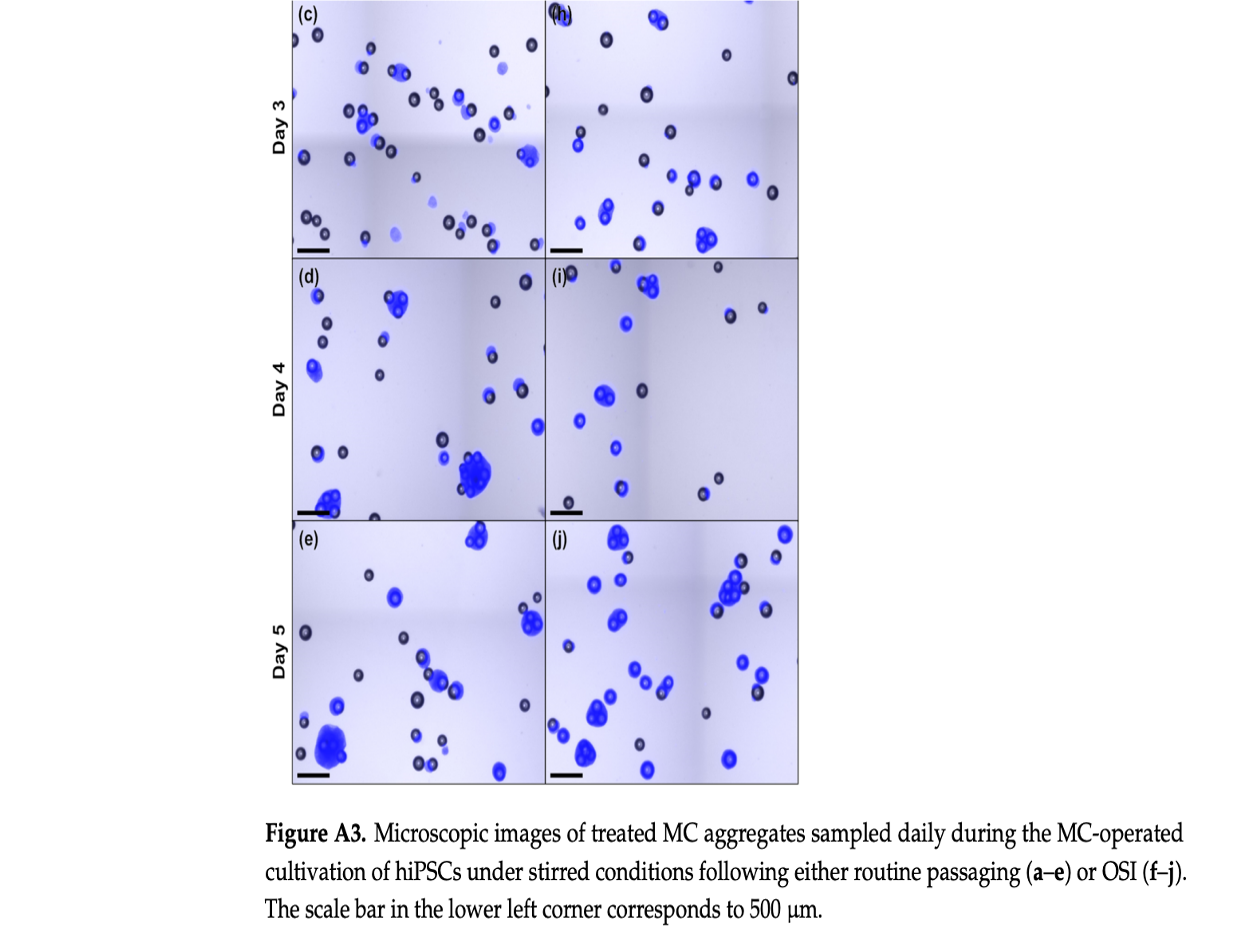
The study revealed that hiPSCs, stored at high densities of up to 100 million cells per milliliter in CryoStor® CS10, maintained their growth potential and quality after thawing. Remarkably, the one-step inoculation method, particularly under dynamic conditions with microcarriers in stirred bioreactors, resulted in significantly improved cell attachment efficiency—about 50% higher within the first day of cultivation compared to traditional methods.
The conclusions of this research highlight the potential of OSI to streamline the hiPSC expansion process, reducing the seed train duration required to produce clinically relevant quantities of these cells. The OSI approach also demonstrated benefits in cell distribution, aggregation, and survivability, with a 35% reduction in cell-specific LDH activity, indicating lower cell stress during the initial stages of cultivation.
This intensified process not only maintained the hiPSCs’ viability, pluripotency, and ability to differentiate into various tissue types but also offered a faster and more efficient route to achieve the large-scale production necessary for therapeutic applications. The study’s findings lay the groundwork for transferring this method to larger-scale manufacturing, potentially revolutionizing the production of hiPSC-based therapies.
For more information on our innovative solutions and to stay updated on the latest developments, visit our website or contact our team at Smart MCs.
Read The Full Artice Here.


Leave A Comment