The FDA has approved Ryoncil (remestemcel-L-rknd), the first mesenchymal stromal cell (MSC) therapy for steroid-refractory acute graft-versus-host disease (SR-aGVHD) in pediatric patients aged two months and older. This approval is a monumental milestone for regenerative medicine, offering a critical treatment option for a condition with limited alternatives and setting the stage for further innovation in the cell therapy industry.
Acute graft-versus-host disease (aGVHD) is a life-threatening complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT), a procedure often used to treat certain blood cancers and disorders. In aGVHD, donor immune cells attack the recipient’s tissues, causing inflammation and damage to critical organs such as the skin, liver, and gastrointestinal tract.
When this condition does not respond to standard steroid treatments, it becomes steroid-refractory (SR-aGVHD), increasing the risk of mortality. For these patients, Ryoncil offers a new therapeutic approach by modulating immune responses to reduce inflammation and improve outcomes.
Ryoncil is an allogeneic therapy derived from the bone marrow of healthy adult donors. Mesenchymal stromal cells are multipotent cells capable of differentiating into various cell types while modulating immune activity, making them particularly effective for inflammatory and immune-related conditions like SR-aGVHD. The therapy works by interacting with T-cells, helping to regulate immune overactivation and reduce organ damage.
The FDA’s approval of Ryoncil is based on a pivotal multicenter, single-arm clinical trial involving 54 pediatric patients with SR-aGVHD. Key findings include:
- A clinically meaningful overall response rate within 28 days of treatment.
- A survival advantage for patients who responded to the therapy.
- An acceptable safety profile, with benefits outweighing the risks of common adverse reactions like infections.
This evidence highlights Ryoncil’s potential as a life-saving treatment for patients with limited alternatives. The approval represents more than just a new treatment; it validates the potential of MSC-based therapies in addressing complex, immune-related diseases. It demonstrates the power of innovative research in regenerative medicine, paving the way for advancements in MSCs, induced pluripotent stem cells (iPSCs), exosomes, and other cell-based technologies.
Ryoncil’s approval has also had a significant impact on the biotechnology sector. Mesoblast, the company behind Ryoncil, experienced a 29% surge in stock value, reflecting investor confidence in MSC-based therapies. This regulatory milestone highlights the growing demand for regenerative medicine solutions and sets a pathway for future therapies to gain FDA approval, encouraging further investment and innovation.
The Need for Scalable Production
As more cell-based therapies approach regulatory approval, the need for scalable, efficient biomanufacturing becomes increasingly urgent. Traditional methods like plastic flasks and roller bottles are insufficient for producing the large volumes of cells required to meet clinical and commercial demands.
At Smart MCs, we specialize in providing microcarrier solutions to help address these challenges. Our platforms enable seamless scalability and optimization for stem cell production.
While Ryoncil used its proprietary production methods, our microcarriers are designed to support other stakeholders in the cell therapy industry with benefits like:
- Effortless Scalability: Scale from 100 mL to thousands of liters.
- Cost Efficiency: Reduce labor and resource requirements.
- Higher Yields: Improve cell retrieval and production outcomes.
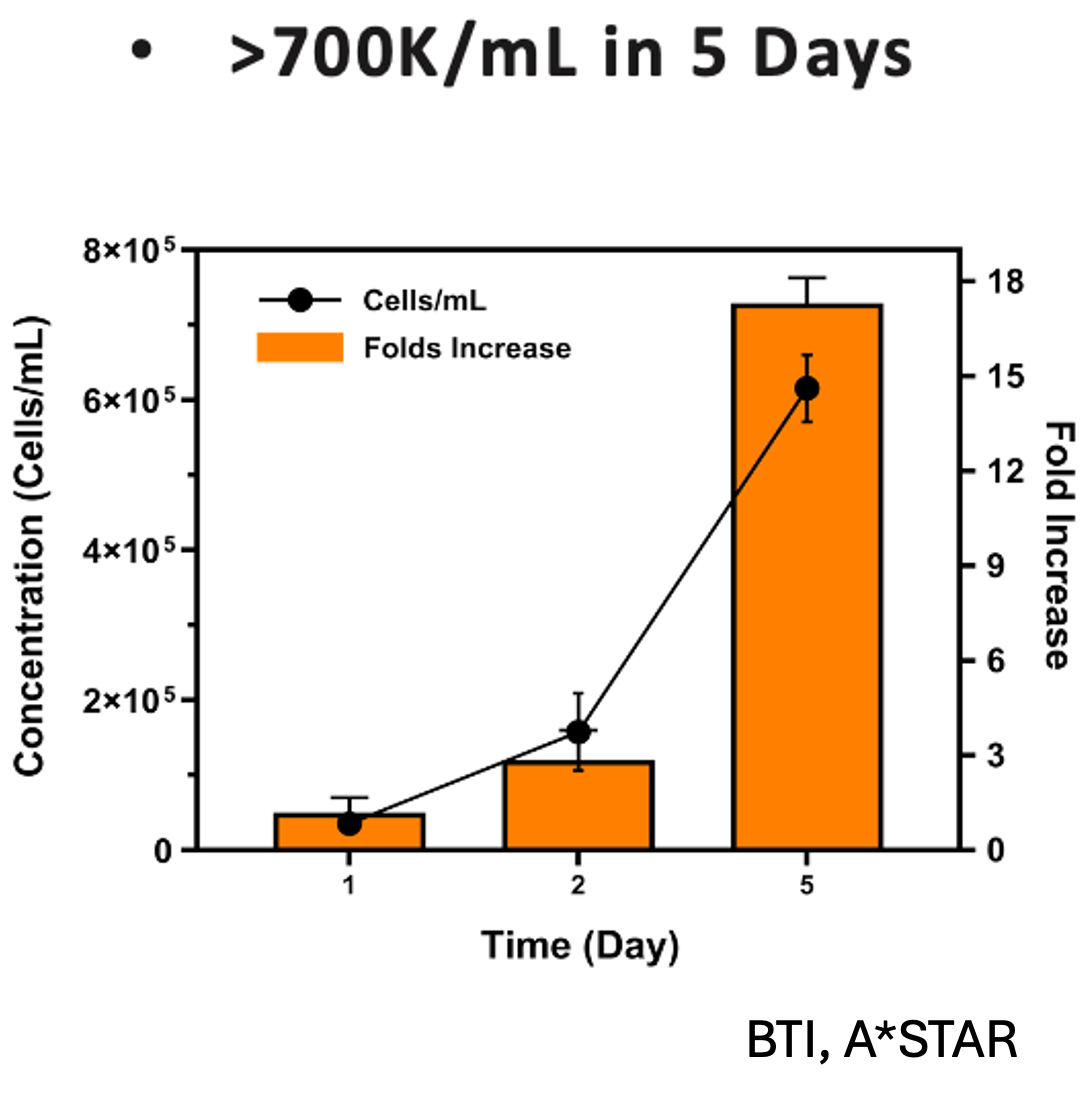
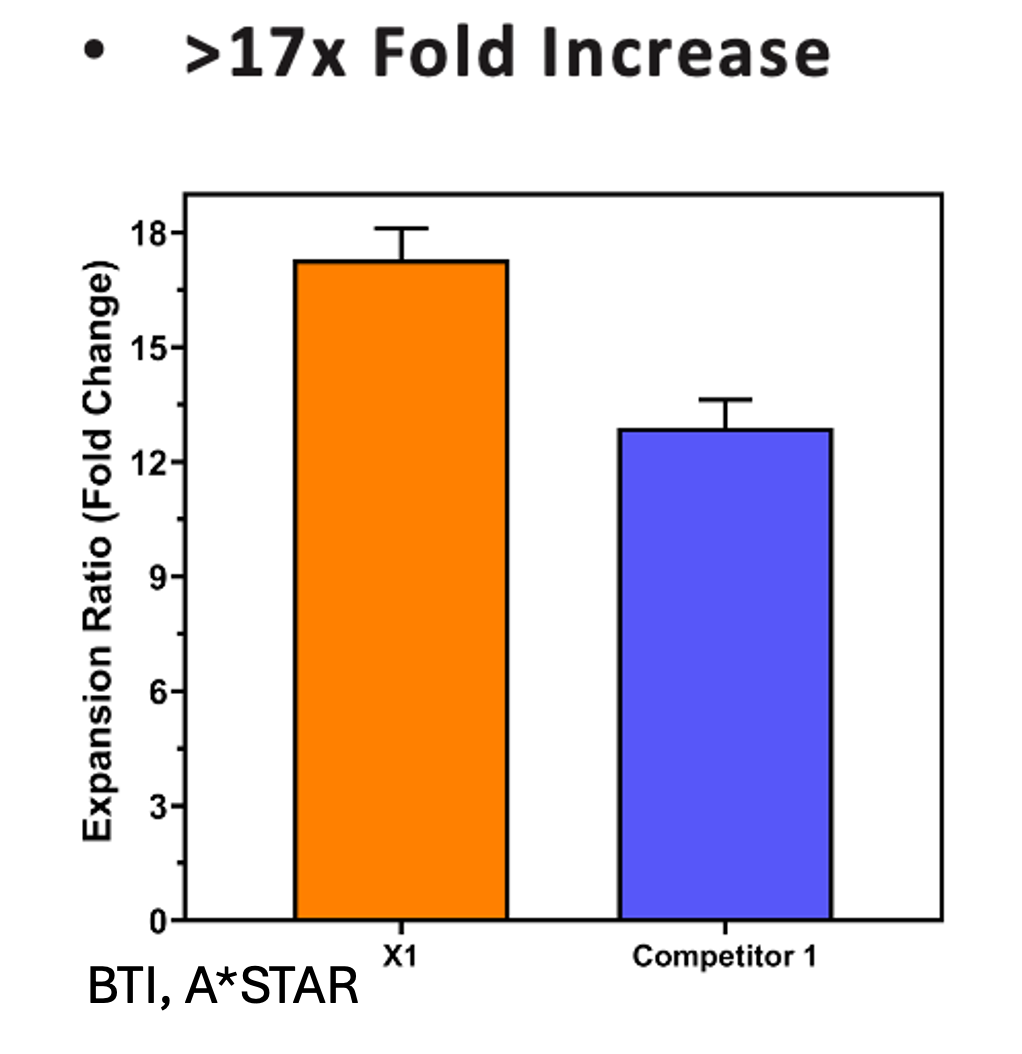
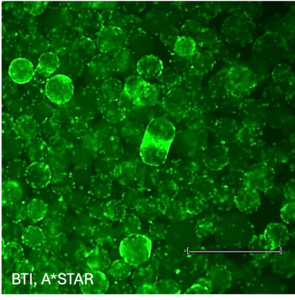 Our flagship product, Smart MCs P2, is a dissolvable and xeno-free microcarrier designed for enhanced scalability. It eliminates the need for costly filtration processes and has demonstrated exceptional performance in independent studies conducted at Bioprocessing Technology Institute (BTI), A*STAR, Singapore, achieving 700,000 cells/mL in just five days with no media changes.
Our flagship product, Smart MCs P2, is a dissolvable and xeno-free microcarrier designed for enhanced scalability. It eliminates the need for costly filtration processes and has demonstrated exceptional performance in independent studies conducted at Bioprocessing Technology Institute (BTI), A*STAR, Singapore, achieving 700,000 cells/mL in just five days with no media changes.
While Mesoblast did not use our microcarriers, Smart MCs solutions are well-positioned to support the broader cell therapy industry in meeting the demands of commercialization.
For companies in the cell therapy space, adopting microcarrier platforms like Smart MCs offers clear advantages:
- Scalability: Seamlessly transition from small-scale research to industrial-scale production.
- Efficiency: Streamline workflows, reduce labor, and achieve higher yields.
- Flexibility: Adapt production processes to meet evolving clinical and commercial needs.
The FDA’s approval of Ryoncil is a landmark moment for cell-based therapies, offering hope for pediatric patients with SR-aGVHD and setting a strong precedent for the future of regenerative medicine. As the cell therapy industry grows, scalable, efficient manufacturing processes will play a vital role in ensuring these life-saving therapies can reach more patients.
At Smart MCs, we are committed to supporting the cell therapy sector with innovative microcarrier solutions. Whether you’re scaling research or moving to full-scale production, we’re here to support your journey in revolutionizing cell therapy.
Ready to revolutionize your stem cell culture processes? Contact Smart MCs today to learn how our microcarrier solutions can help you achieve your production goals or check out our product page.
References:
Investors Business Daily. (2024). Mesoblast biotech stock skyrockets on graft-versus-host disease approval. Retrieved December 19, 2024, from Link
U.S. Food and Drug Administration. (2024). FDA approves first mesenchymal stromal cell therapy to treat steroid-refractory acute graft-versus-host disease. Retrieved December 19, 2024, from Link
U.S. Food and Drug Administration. (2024). FDA approves remestemcel-L-rknd for steroid-refractory acute graft-versus-host disease in pediatric patients. Retrieved December 19, 2024, from Link






Leave A Comment