That’s why scientists have developed what’s called a quadrivalent vaccine, a vaccine designed to protect against all four dengue virus types at once.
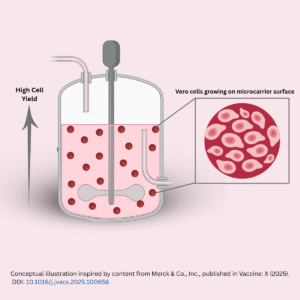
But making this kind of vaccine on a large scale is not simple. It requires growing viruses safely and consistently in laboratory conditions. This blog explains how scientists used a combination of Vero cells, microcarriers, and bioreactors to improve the manufacturing process for a live dengue vaccine, while making it more scalable and efficient.
This study was conducted by researchers at Merck & Co., Inc. and published in Vaccine: X in May 2025 under the title “Optimization for the production of a dengue live-attenuated quadrivalent vaccine in Vero cells grown on microcarriers.” The team applied a structured design-of-experiment (DoE) approach to optimise virus production using Vero cells on microcarriers, demonstrating scalable and efficient manufacturing from 2L to 50L bioreactors. The full paper is available open access at https://doi.org/10.1016/j.jvacx.2025.100658.
How the Vaccine Is Made
To create this vaccine, researchers use a weakened (live-attenuated) version of each dengue virus type. These are grown in the lab inside Vero cells, which are cells originally derived from the kidney of an African green monkey and are commonly used in vaccine production.
However, Vero cells need a surface to grow on. Instead of using flat containers like flasks, the team used microcarriers, tiny beads that float in liquid and provide surface area for cells to grow in bioreactors (large, controlled vessels used for cell culture). This allows the process to be scaled up more easily and consistently.
What Are Microcarriers, and Why Do They Matter?
Microcarriers are tiny, solid beads suspended in liquid culture medium. They serve as a surface for cells to attach and grow especially useful for anchorage-dependent cell lines like Vero cells, which cannot grow freely in suspension.
In this study, microcarriers enabled:
- High-density cell growth in bioreactors
- Efficient virus production from each dengue serotype
- Scalability of the process to larger volumes (up to 50 litres)
- Improved process control, such as even distribution of nutrients and oxygen
By shifting from traditional flat-surface cultures (like flasks) to microcarrier-based systems, the process becomes much more suitable for large-scale, consistent vaccine production.
What the Study Tested
The researchers looked at four main factors to improve the virus production process using Vero cells cultured on microcarriers:
- Temperature – How warm the culture environment is.
- pH – How acidic or basic the liquid is.
- Multiplicity of Infection (MOI) – How many virus particles are added to infect the cells.
- Time of Infection (TOI) – When the virus is added during the cell growth phase.
They ran experiments using small bioreactors (2–3 litres), then tested their best conditions in a 50-litre single-use bioreactor, a size relevant to industrial production.
Key Findings
- Best temperature and pH: 34°C and pH 7.0 led to the highest virus production across all four dengue types.
- Significant increase in output: Some dengue virus types produced up to 10 times higher yields than under baseline conditions.
- Flexible process: Changing when or how much virus was added didn’t make much difference to results, good news for manufacturing.
- Better quality virus: The final virus had more infectious particles and fewer defective ones.
Overview of Results
| Parameter | Outcome |
| Cell system | Vero cells on microcarriers |
| Optimal virus production | 34.0 °C, pH 7.0 |
| Scalability | Confirmed at 2L, 3L, and 50L bioreactors |
| Best yield (DENV1) | Up to 1.09 × 10⁸ PFU/mL |
| MOI/TOI flexibility | No significant impact on peak yield |
| Virus quality | Higher infectivity; fewer defective particles |
What This Means for Global Vaccine Manufacturing
The production of live-attenuated vaccines, like the quadrivalent dengue candidate in this study, depends on the ability to generate high volumes of virus with consistent quality. Microcarrier-based systems are central to making this happen.
Why this matters:
- High-throughput production: Large batches of virus can be made in smaller spaces.
- Consistency and control: Bioreactors with microcarriers allow fine-tuning of temperature, pH, oxygen, and other parameters.
- Readiness for scale-up: Once conditions are optimised at small volumes, they can be replicated at industrial scale more reliably.
This approach benefits not only dengue vaccine development but also other live-virus vaccines requiring Vero cell platforms.
Conclusion
This study demonstrates that a standardised microcarrier-based system, combined with strategic optimisation of production parameters, can significantly enhance virus yield and manufacturing consistency for a quadrivalent dengue vaccine.
By enabling reliable scale-up to 50-litre bioreactors, this work brings live-attenuated dengue vaccine production closer to meeting global demand especially in regions where dengue is endemic.
Microcarriers are not just a component of the system; they are essential to unlocking the scale, efficiency, and control needed for modern viral vaccine manufacturing.
Microcarriers in Context
This study showcases how microcarrier-based systems play a critical role in scalable virus vaccine production.
Disclaimer: This blog discusses a study conducted by Merck & Co., Inc., which used a different microcarrier product not associated with Smart MCs. The findings reflect the broader utility of microcarrier-based systems in vaccine manufacturing. Smart MCs offers its own line of microcarriers developed to support consistent, scalable performance in similar bioreactor-based applications. Mention of this study is for informational purposes only and does not imply endorsement of Smart MCs by the authors or their institutions.



Leave A Comment