In a recent study, researchers have developed a novel microcarrier-microbioreactor platform that shows significant promise for improving the manufacturing process of mesenchymal stem and stromal cells (MSCs), which are vital for treating a range of clinical conditions. Despite the potential of MSCs, their clinical application has been hindered by challenges in achieving consistent product quality, particularly concerning critical quality attributes (CQAs) that are essential for predicting the potency and efficacy of these cells.
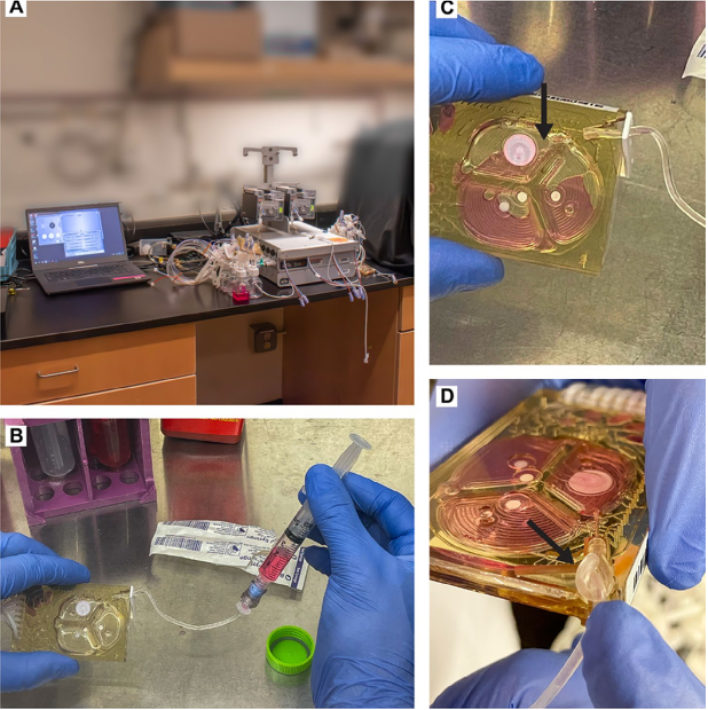
The research team focused on addressing these challenges by designing a microcarrier-microbioreactor system tailored for anchorage-dependent cells like MSCs. Their goal was to assess whether this approach could provide better control over the biochemical and biophysical environment, leading to more consistent and higher expression of potential critical quality attributes (pCQAs) compared to traditional methods using tissue culture polystyrene (TCPS) flasks.
In their proof-of-concept study, the researchers evaluated cell yield, gene expression, and protein translation in MSCs cultured within their innovative system. They discovered that MSCs expanded using the microcarrier-microbioreactor platform not only showed consistent donor-to-donor and batch-to-batch reproducibility but also exhibited significantly improved expression of key pCQAs at both the gene and protein levels. Notably, this approach also reduced media consumption, highlighting its efficiency over conventional TCPS culture methods.
The study specifically targeted MSCs for the treatment of acute respiratory distress syndrome (ARDS), a condition with limited effective therapies. The researchers identified and analyzed 24 genes linked to therapeutic efficacy for ARDS, using them as pCQAs to evaluate the potency of the MSCs produced. The results revealed markedly increased expression of many pCQAs in the cells cultured with the microcarrier-microbioreactor system, suggesting enhanced therapeutic potential.
The research also demonstrated that the system could efficiently regulate crucial biochemical variables such as temperature, pH, and CO2 levels, ensuring optimal conditions for MSC growth and function. Additionally, the custom-designed gelatin microcarriers facilitated efficient cell harvest and provided mechanical cues that mimic physiological conditions, further enhancing the cells’ therapeutic attributes.
While the results are promising, the researchers acknowledge that further process optimization is necessary, particularly to manage the increased expression of pro-inflammatory pCQAs observed in some cases. However, the overall findings underscore the potential of this innovative approach to revolutionize MSC manufacturing, offering a scalable, resource-efficient, and quality-driven solution for producing therapeutic cells.
This study represents a significant step forward in cell therapy manufacturing, with broader implications for other anchorage-dependent cell types and therapeutic applications. The microcarrier-microbioreactor platform offers a powerful tool for advancing MSC-based therapies, particularly in conditions like ARDS, where traditional treatments have proven inadequate. Future research will focus on refining the system to further enhance product consistency and therapeutic efficacy, paving the way for successful clinical translation of MSC therapies.




Leave A Comment