The United States Food and Drug Administration (FDA) recently announced a significant policy change. The FDA plans to phase out its requirement for animal testing in the safety evaluation of certain medicines, including monoclonal antibodies (mAbs). This change reflects the FDA’s effort to update drug testing practices with methods that are more relevant to human biology.
For researchers, pharmaceutical companies, and biotechnology organisations, this update marks an important shift in how new medicines may be tested and approved in the years ahead.

What Are Monoclonal Antibodies?
Monoclonal antibodies (mAbs) are laboratory-made proteins designed to target specific molecules in the body.
They work by attaching to a particular cell or protein — often a disease-causing target — and either blocking its action or signalling the immune system to remove it.
Monoclonal antibodies are now used to treat a wide range of medical conditions, including:
- Cancer
- Autoimmune diseases (such as rheumatoid arthritis or multiple sclerosis)
- Infectious diseases (including COVID-19)
- Allergic conditions
Because they interact directly with human cells, evaluating their safety is a critical part of drug development.
Traditionally, this has involved animal studies. However, the FDA is moving towards more modern testing strategies.
What is NAM?
NAM stands for New Approach Methodologies.
This term refers to scientific methods that can replace, reduce, or refine the use of animals in drug testing. The aim of NAM is to gather safety and effectiveness data using human-relevant approaches.
These may include:
- Laboratory testing on human cells or tissues
- Computer modelling and simulations
- Analysis of real-world human data from clinical use
The FDA’s updated policy encourages drug developers to use NAM approaches where appropriate, particularly when they can provide better information about human safety than traditional animal studies.
Why Is This FDA Policy Change Important?
This update is part of a broader shift in pharmaceutical research and regulation.
Animal studies have been a longstanding part of drug development, but they have limitations. A medicine that is safe in animals may not always produce the same results in humans.
Moving towards NAM approaches offers several clear benefits:
✔️ Improved Human Safety Predictions. Methods based on human cells or data can provide more accurate information about how a medicine will work in people.
✔️ Faster Development Timelines. Reducing reliance on animal studies may help identify safety concerns earlier in the development process, potentially shortening the time required for clinical trials.
✔️ Ethical Considerations. Minimising animal use aligns with growing expectations for more responsible and humane research practices.
✔️ Global Alignment. Other regulatory agencies, including those in Europe and Australia, are also encouraging the adoption of NAM where appropriate. The FDA’s update supports international consistency in drug development standards.
You can read the full FDA announcement here.
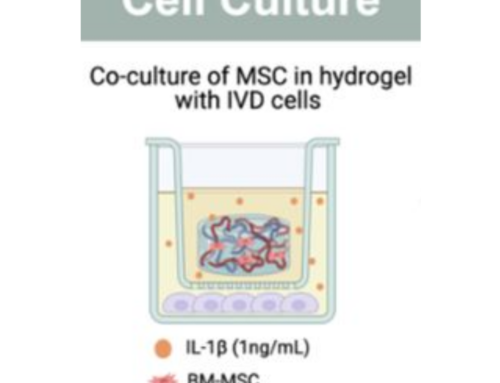
How Hydrogels Support Human-Relevant Testing in the Lab
As the use of human-relevant testing methods grows, materials like hydrogels are playing an increasingly important role in laboratory research.
Hydrogels provide a 3D environment for growing human cells, allowing researchers to build tissue models that better replicate how the human body responds to medicines. These advanced lab models help support the development of safer and more effective drug testing systems.
Hydrogels are soft, water-based materials that create a three-dimensional structure for growing human cells in the laboratory. This makes them ideal for developing advanced tissue models used in NAM approaches.
Hydrogels help researchers:
- Grow human cells in 3D environments
- Build laboratory models of human tissues or organs
- Study medicine responses in systems that more closely represent the human body
As the industry moves away from animal testing, hydrogels will continue to play a key role in supporting research that is safer, faster, and more human-relevant.
Need Hydrogel for Your Research?
As drug development moves towards faster and more human-relevant testing methods, having the right research materials is essential.
We supply high-quality hydrogels designed for biomedical research, medicine testing, and tissue engineering.
We are here to support your research with materials that help build safer and more human-relevant testing systems.




Leave A Comment