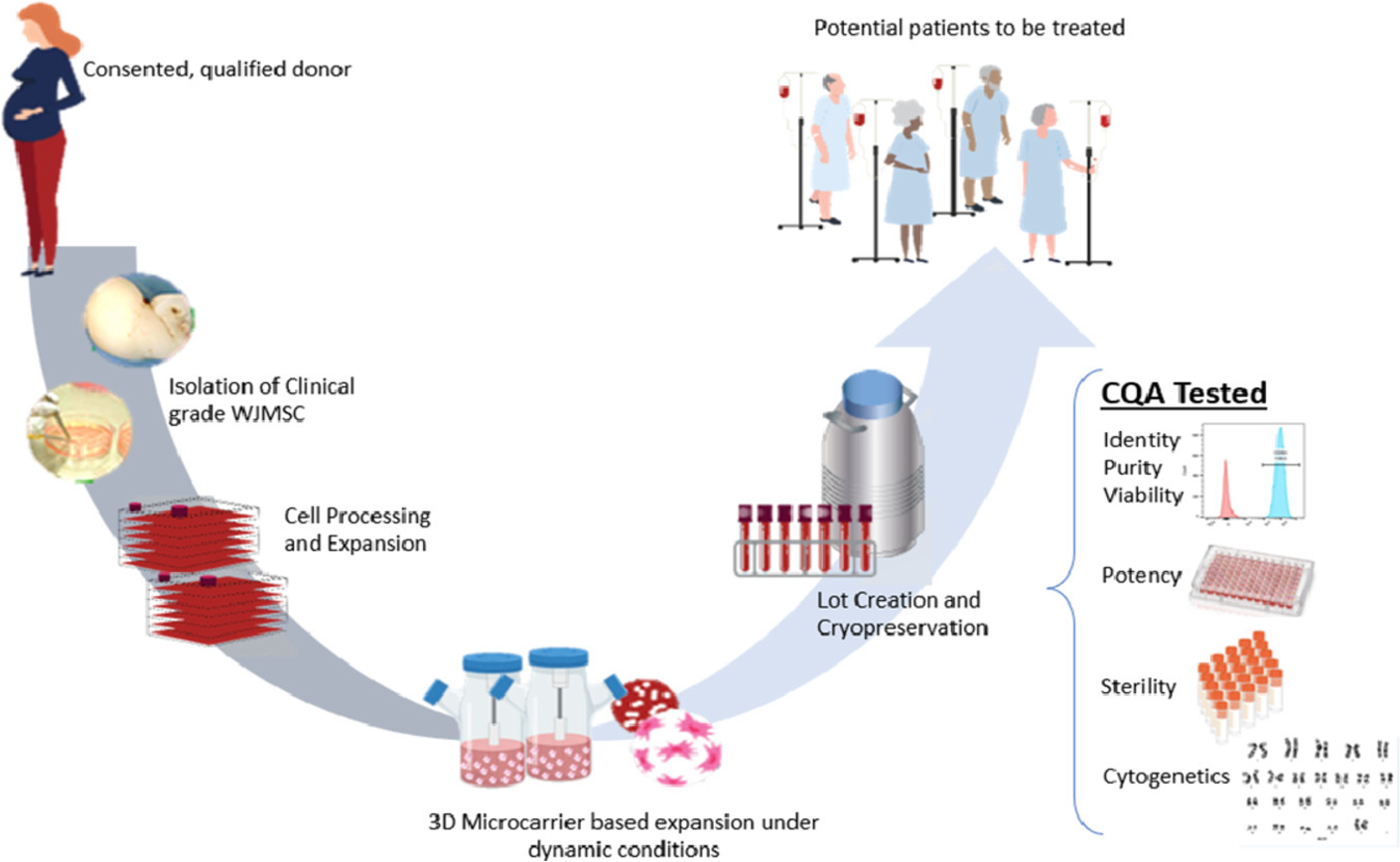
Wharton’s jelly mesenchymal stem/stromal cells (WJMSCs) have gained significant attention due to their potential in immunomodulatory, anti-inflammatory, tissue repair, and regenerative therapies. These cells have shown promise in treating various clinical conditions, highlighting the need for scalable manufacturing processes to meet commercial and clinical demands. Recent research has focused on developing a robust, scalable, and current Good Manufacturing Practice (cGMP)-compliant method for the expansion of WJMSCs.
In a pivotal study, researchers optimized culture parameters such as microcarrier type, seeding density, agitation, and culture feed regime using a 3D microcarrier-based culture system in spinner flasks. This approach aimed to transition from a traditional 2D planar culture system to a more efficient 3D suspension process, achieving higher cell yields while maintaining cell quality and functionality.
Key Findings
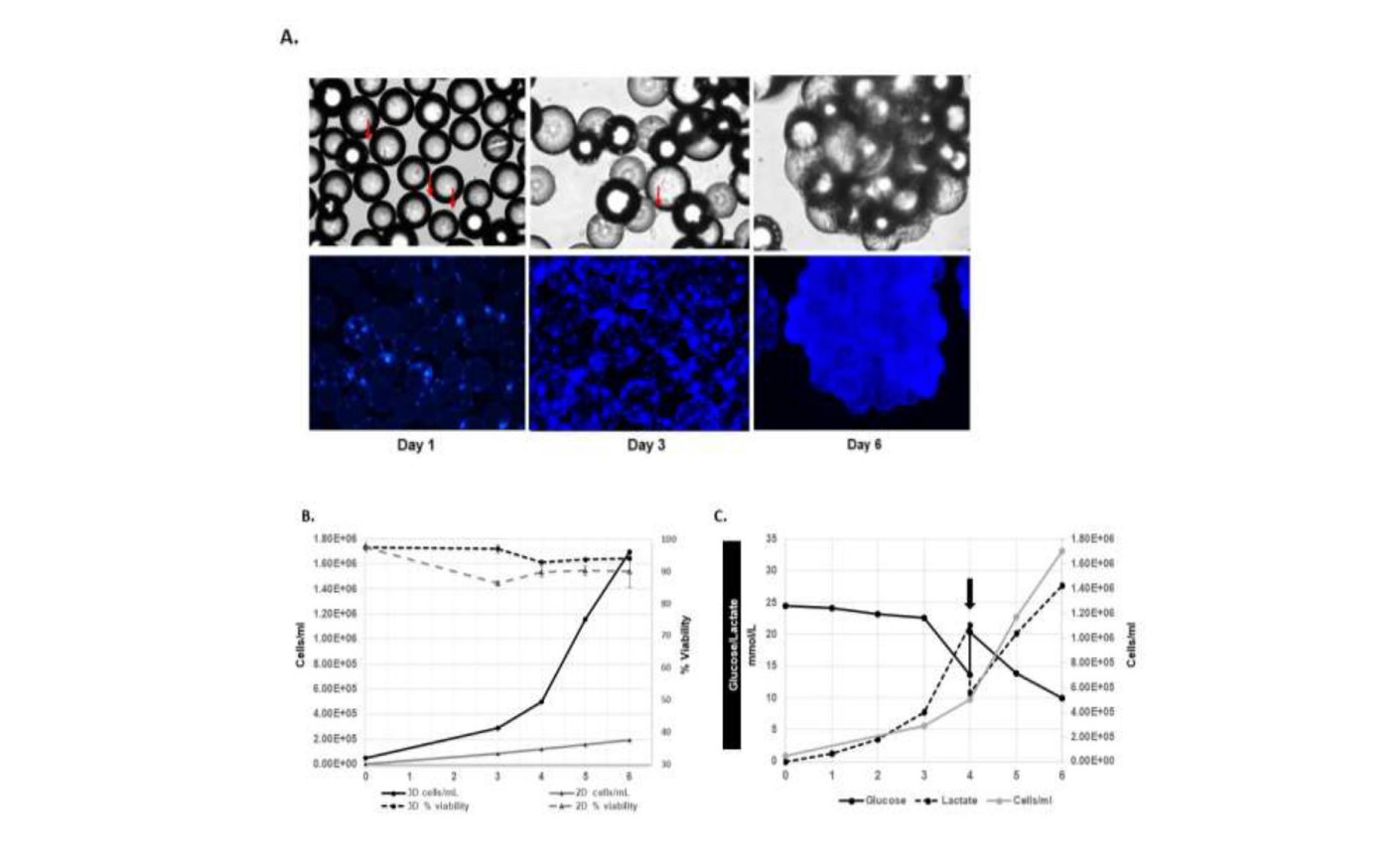
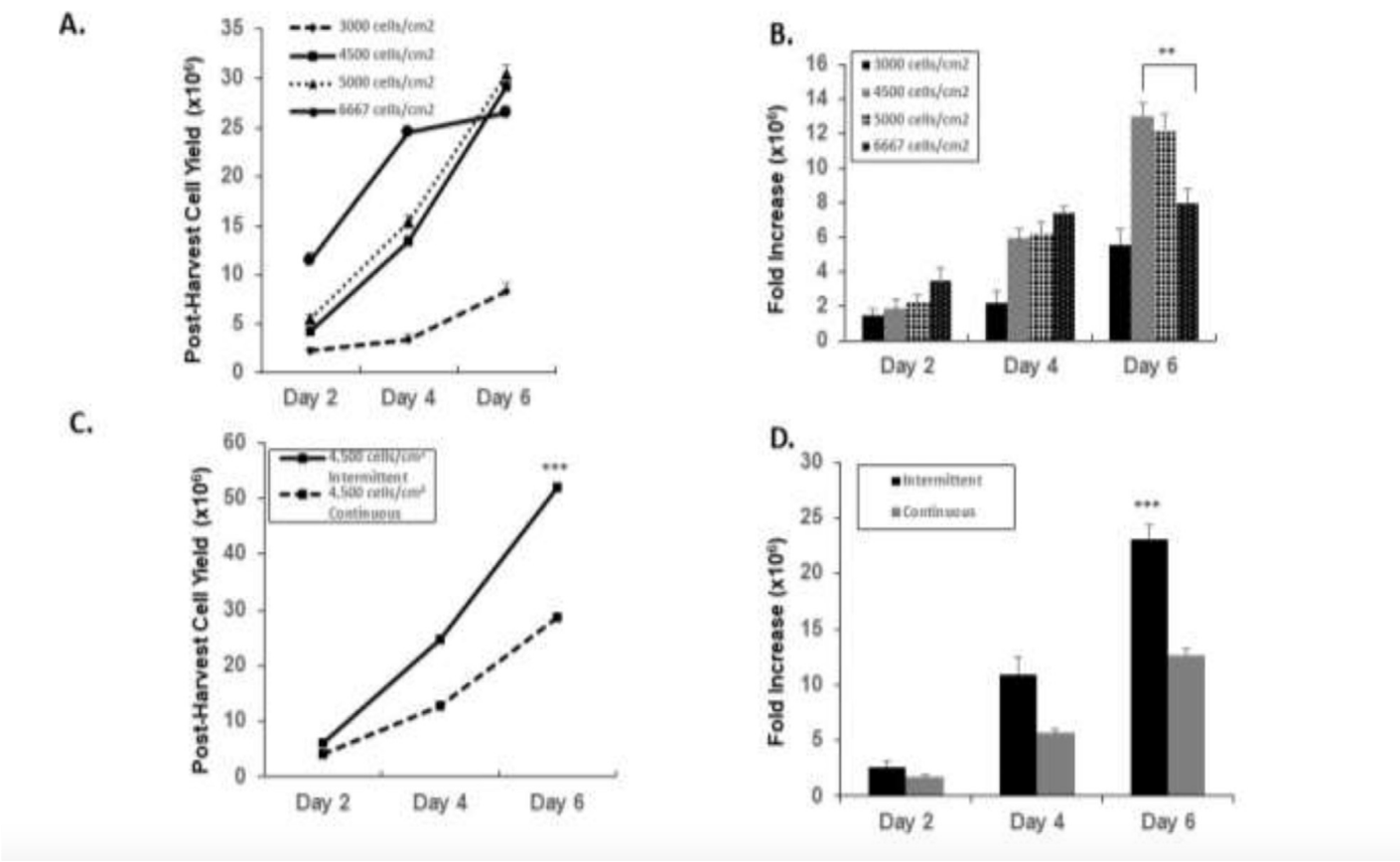
The study screened various commercially available microcarriers to identify the optimal type for WJMSC expansion. Collagen-coated microcarriers emerged as the superior choice, facilitating significant cell proliferation and achieving a cell density of 4.26 × 10^5 cells/mL and a post-harvest yield of 0.85 × 10^6 cells with over 95% viability. This result corresponded to a 6.5-fold expansion, significantly outperforming other microcarriers tested.
Further optimization in spinner flasks showed that intermittent stirring (3 minutes at 25 rpm followed by 30 minutes of non-agitation) and reduced supplementation during the initial 8 hours of cultivation were critical for enhancing cell adhesion and growth. This method achieved a maximum cell density of 1.67 × 10^6 cells/mL, yielding approximately 84 × 10^6 cells after 6 days of culture with a 95% harvest efficiency.
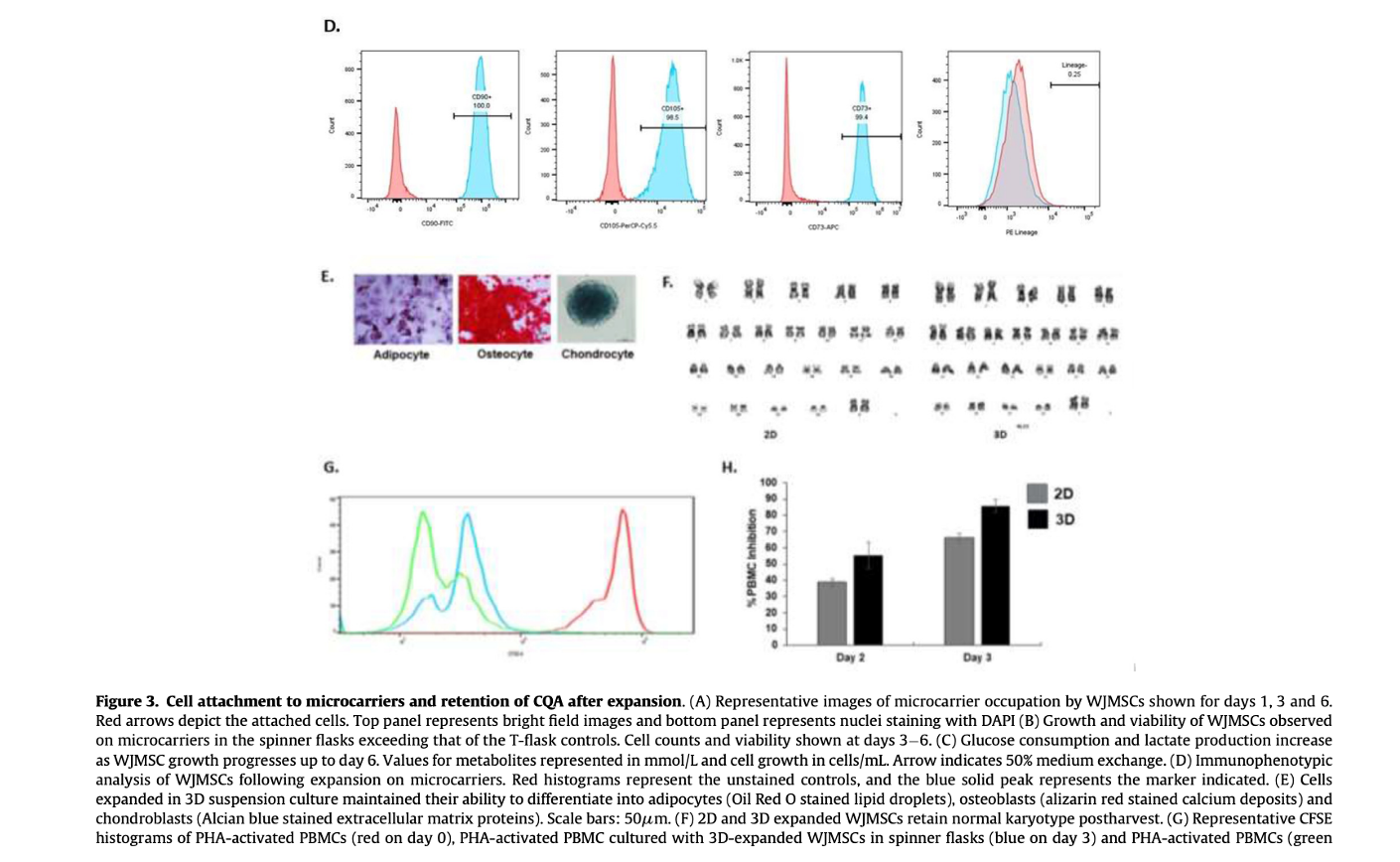
Importantly, post-expansion analyses confirmed that the WJMSCs retained their phenotypic characteristics, differentiation potential, normal karyotype, functional properties, and sterility, ensuring their suitability for clinical applications.
Discussion
The shift from 2D to 3D culture systems offers several advantages, including higher cell yields, cost-effectiveness, and reduced labor and time requirements. The study’s success in establishing a cGMP-compliant, scalable expansion process for WJMSCs marks a significant step towards meeting the commercial demands for clinical-grade mesenchymal stromal cells.
The findings underscore the importance of selecting appropriate microcarriers and optimizing culture conditions to achieve efficient and reproducible cell expansion. The use of collagen-coated microcarriers, in particular, provided a conducive environment for WJMSC growth, likely due to their ability to mimic the in vivo extracellular matrix.
Future research will focus on scaling up this process in larger bioreactors and conducting detailed economic analyses to ensure the viability and cost-effectiveness of xeno-free bioprocesses. Additionally, studies will explore the long-term effects of over-confluency on MSC functions to further optimize and refine the expansion process.
Conclusion
The study presents a groundbreaking approach to large-scale WJMSC expansion using a 3D microcarrier-based suspension culture system. This method not only meets the stringent cGMP requirements but also achieves high cell yields while maintaining cell quality and functionality. The successful transition from 2D to 3D culture systems represents a significant advancement in the field of cellular therapy, paving the way for the commercial production of clinical-grade mesenchymal stromal cells.
For more information on our innovative solutions and to stay updated on the latest developments, visit our website or contact our team at Smart MCs.
Read The Full Artice Here.




Leave A Comment