The following protocols for isolating Mesenchymal Stem Cells (MSCs) are adapted and simplified by FUJIFILM Irvine Scientific from published reports. Please note that these protocols have not been validated by Smart MCs and are provided for reference purposes only. For additional MSC-related protocols and reagents, please visit the FUJIFILM Irvine Scientific website.
You can view the full protocol on FUJIFILM Irvine Scientific’s website here: Protocol for Mesenchymal Stem Cell Isolation.
Mesenchymal Stem Cell Isolation from Adipose Tissue – Rapid Method
- Collect the sample: Aspirate blood/saline into a 50mL conical tube.
- Centrifuge: Spin the sample at 400xg for 10 minutes at room temperature.
- Wash the pellet: Resuspend the pellet in 160mM NH4Cl and incubate for 5 minutes at room temperature to remove red blood cells.
- Centrifuge again: Spin the sample at 400xg for another 10 minutes.
- Remove the supernatant: Discard the supernatant and resuspend the pellet in 40% FBS/DMEM, then plate the cells.
- Incubate overnight: Place the plate in a 37°C incubator with 5% CO2 overnight.
Mesenchymal Stem Cell Isolation from Adipose Tissue – Standard Method
- Remove saline and oil: Aspirate off the saline and oil layers from the fat tissue.
- Wash the fat: Rinse approximately 250mL of fat tissue 3-5 times with PBS, discarding the lower phase until the wash is clear.
- Add collagenase: Add collagenase to the fat tissue and incubate for 1-4 hours at 37°C with gentle shaking.
- Neutralise the collagenase: Add 10% FBS to neutralise the enzyme.
- Centrifuge the sample: Spin the sample at 800xg for 10 minutes to separate the components.
- Aspirate lipids: Discard the floating adipocytes, lipids, and liquids, leaving behind the stromal vascular fraction (SVF) pellet.
- Resuspend the pellet: Resuspend the SVF pellet in 160mM NH4Cl and incubate for 10 minutes at room temperature.
- Centrifuge again: Spin the sample at 400xg for 10 minutes to remove remaining contaminants.
- Layer on a gradient: Layer the cells onto a Percoll or Histopaque gradient and centrifuge at 1000xg for 30 minutes.
- Wash the cells: Wash the cells twice with PBS, centrifuging at 400xg for 10 minutes between washes.
- Filter the cell suspension: Pass the cells through a 100µM nylon mesh filter to remove clumps.
- Refine with 400µM mesh: Pass the cells through a 400µM mesh for further purification.
- Final centrifuge: Centrifuge the cells at 400xg for 10 minutes to concentrate them.
- Plate the cells: Resuspend the pellet in 40% FBS/DMEM and plate the cells.
- Incubate overnight: Incubate the cells at 37°C with 5% CO2 overnight.
Mesenchymal Stem Cell Isolation from Cord Blood
- Dilute the cord blood: Mix the cord blood with RPMI Medium 1640 at a 3:1 ratio (3 parts cord blood to 1 part RPMI).
- Perform density gradient centrifugation: Use Ficoll-Paque™ Premium to isolate the mononuclear cells (MNCs) by centrifuging at 400xg for 30 minutes, following the manufacturer’s instructions.
- Wash the MNCs: Transfer the MNCs into a new tube and dilute with PBS at a 1:3 ratio (1 part MNCs to 3 parts PBS).
- Centrifuge: Spin the sample at 400xg for 10 minutes at room temperature.
- Remove supernatant: Discard the supernatant, resuspend the cells in culture medium, and plate the cells.
- Incubate overnight: Incubate the cells at 37°C in a 5% CO2 incubator overnight.
Note: Ficoll-Paque™ is a registered trademark of GE Healthcare.
Mesenchymal Stem Cell Isolation from Umbilical Cord
- Prepare the cord: Wash the umbilical cords in a 1:3 hypochlorite solution, then rinse with PBS.
- Storage: Store the cords in 10% FBS/DMEM-low glucose medium for up to 12 hours before processing.
- Inject with collagenase: Inject the veins and arteries of the cord with 3mL of 0.1% collagenase in PBS.
- Incubate the tissue: Let the cord incubate for 20 minutes at 37°C.
- Wash and inject further: After incubation, inject the tissue with 5mL of DMEM-low glucose medium with 10% FBS.
- Harvest the cells: Gently massage the cord tissue to release cells.
- Centrifuge: Spin the sample at 300xg for 10 minutes at room temperature.
- Remove supernatant: Discard the supernatant, add fresh culture medium, and plate the cells.
- Incubate overnight: Place the plate in a 37°C incubator with 5% CO2 overnight.
Credit
This protocol is adapted from FUJIFILM Irvine Scientific, “Protocol for Mesenchymal Stem Cell Isolation,” available at https://www.irvinesci.com/protocol-for-mesenchymal-stem-cell-isolation.
- Francis, M. P., Sachs, P. C., Elmore, L. W., & Holt, S. E. (2010). Isolating adipose-derived mesenchymal stem cells from lipoaspirate blood and saline fraction. In Organogenesis (Vol. 6, Issue 1, pp. 11–14). Informa UK Limited. https://doi.org/10.4161/org.6.1.10019
- Secco, M., Zucconi, E., Vieira, N. M., Fogaça, L. L. Q., Cerqueira, A., Carvalho, M. D. F., Jazedje, T., Okamoto, O. K., Muotri, A. R., & Zatz, M. (2007). Multipotent Stem Cells from Umbilical Cord: Cord Is Richer than Blood! In Stem Cells (Vol. 26, Issue 1, pp. 146–150). Oxford University Press (OUP). https://doi.org/10.1634/stemcells.2007-0381
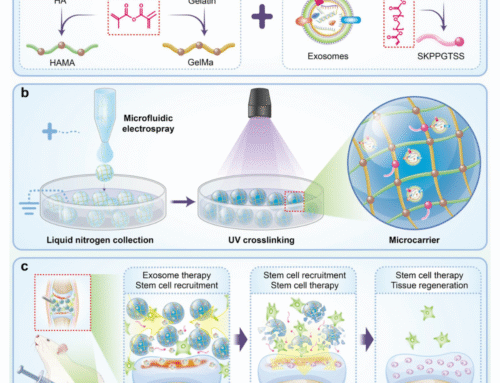
Cell Culture Using Microcarriers
After isolating and plating the MSCs, you can proceed with culturing the cells using microcarriers to expand them. Microcarriers are ideal for large-scale cell culture, providing a larger surface area for cell attachment, which is especially useful for MSC expansion.
Microcarrier-based culture can be performed in bioreactors or flasks, where cells adhere to the microcarriers and proliferate. This method is commonly used for expanding MSCs in research or clinical applications.
Need microcarriers? Check out our product page or feel free to contact us at info@smartmcs.com.au for more information.







Leave A Comment