A recent study by Ulpiano et al., published in Stem Cell Research & Therapy (2025), presents a fully scalable, Good Manufacturing Practice (GMP)-compliant platform for producing extracellular vesicles (EVs) derived from human mesenchymal stromal cells (MSCs). The platform uses microcarrier-based stirred-tank reactors (STRs) combined with advanced purification methods to enable continuous, large-scale production of clinical-grade MSC-EVs under xenogeneic-free conditions.
Background: Why MSC-Derived Extracellular Vesicles Matter
Extracellular vesicles are tiny, membrane-bound particles secreted by cells that carry proteins, RNA, and signalling molecules. MSC-derived EVs have demonstrated powerful roles in tissue repair, immune regulation, and cell-to-cell communication. Unlike whole-cell therapies, EVs avoid risks such as immune rejection, do not replicate, and are easier to store and handle, making them promising candidates for off-the-shelf therapeutic products.
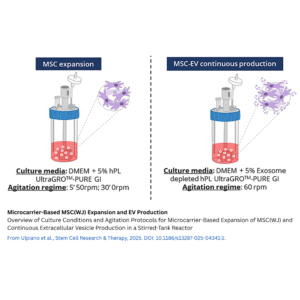
Culturing MSCs at Scale with Microcarrier-Based Stirred-Tank Reactors
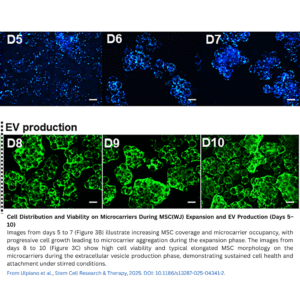
The study focused on MSCs derived from Wharton’s Jelly (MSC(WJ)) of the human umbilical cord, which are known to have higher expansion rates and EV productivity compared to other MSC sources. To grow these cells at large scale, the researchers used dissolvable microcarriers inside a controlled STR system. Microcarriers provide a large surface area in a three-dimensional environment, allowing cells to attach and proliferate efficiently while enabling automated control of culture conditions such as pH, temperature, and oxygen.
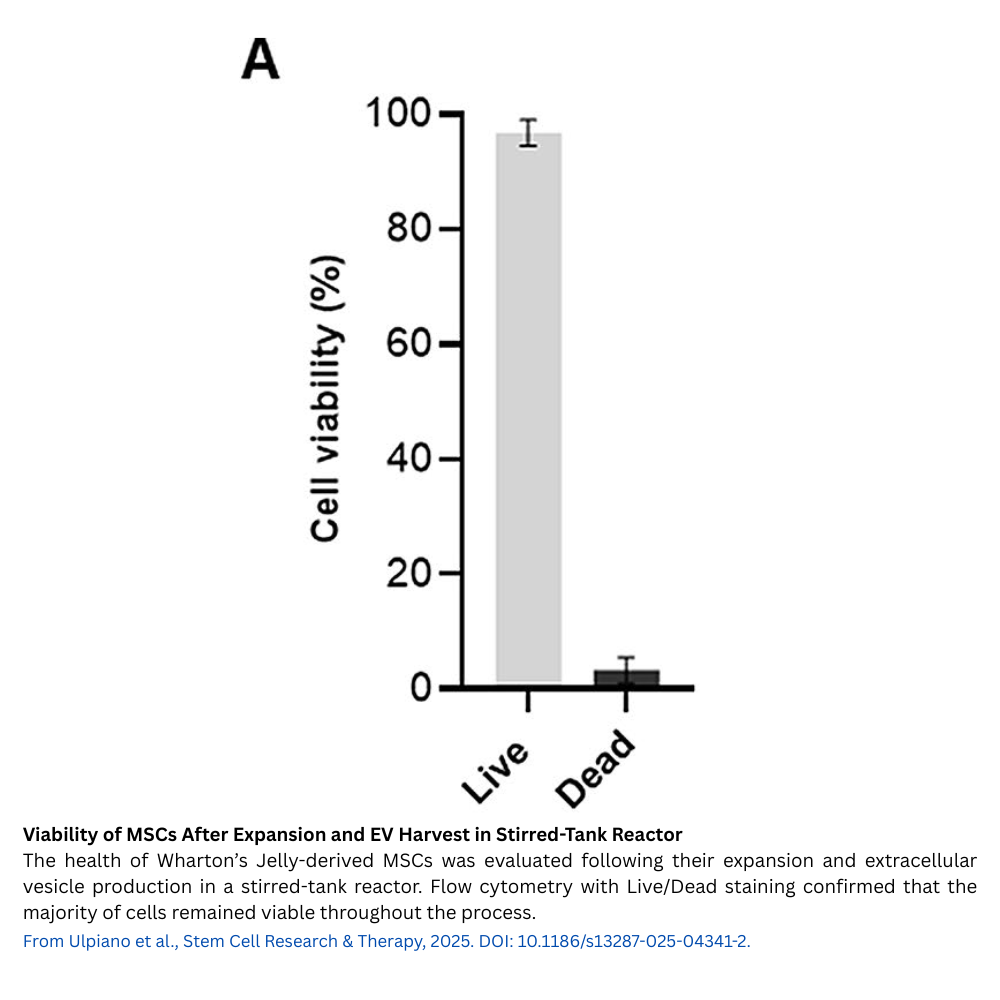
MSC(WJ) were seeded at about 1,850 cells/cm² on coated dissolvable microcarriers. Over 7 days of expansion in medium supplemented with human platelet lysate (hPL) — depleted of exosomes to avoid contamination — the cells grew approximately 30-fold, reaching about 6 × 10^7 cells with over 96% viability. Intermittent agitation and a carefully controlled feeding strategy maintained glucose and lactate at optimal levels to support healthy growth.
Continuous Extracellular Vesicle Collection
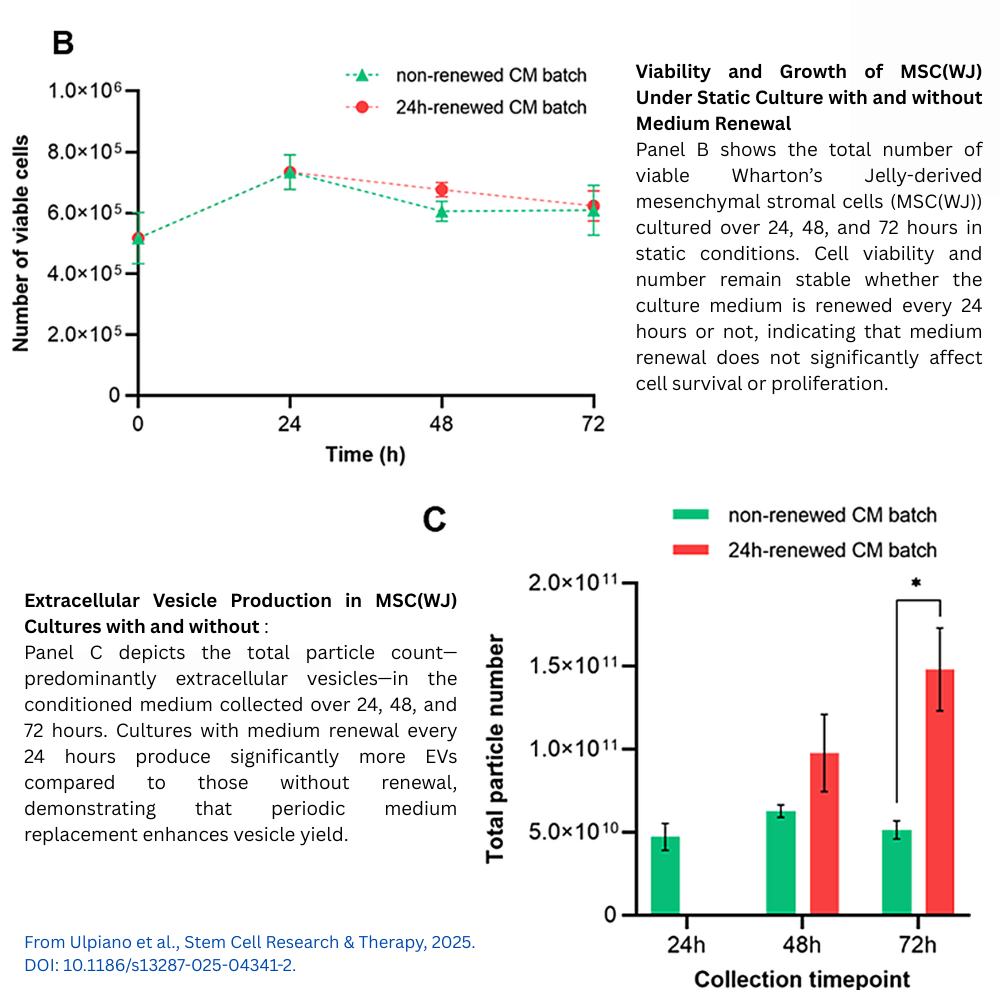
After expansion, MSC(WJ)-laden microcarriers were washed and cultured for 3 days in an EV-depleted, xenogeneic-free hPL medium under continuous stirring at 60 rpm. Conditioned medium enriched in EVs was continuously harvested via perfusion, yielding about 2.13 × 10^12 EVs in total. The particle secretion rate remained stable throughout the collection period, suggesting that this continuous harvesting approach effectively maintains EV production without harming cell viability or function.
Scalable, High-Purity EV Isolation
To isolate EVs from the large volumes of conditioned medium, the team used a scalable downstream process combining tangential flow filtration (TFF) for concentration and buffer exchange, followed by anion exchange chromatography (AEC) for purification. This approach overcomes the limitations of traditional ultracentrifugation by delivering higher purity and scalability.
The isolated EVs exhibited a typical size distribution centred around 115 nm, displayed the expected cup-shaped morphology under electron microscopy, and expressed classical EV surface markers CD9, CD63, and syntenin-1, while lacking cellular contaminants such as calnexin. The particle-to-protein ratio indicated a high purity product suitable for clinical applications.
Functional Validation
To confirm biological functionality, fluorescently labelled MSC-EVs were incubated with human endothelial cells (HUVECs) and breast cancer cell lines (MDA-MB-231 and MCF-7). The EVs were readily internalised by all tested cells, supporting their potential to deliver therapeutic cargo and interact with target tissues.
Conclusion: A Robust Platform for Clinical-Grade MSC-EV Manufacturing
This study establishes a robust, GMP-compliant manufacturing platform integrating microcarrier-based STR expansion of MSC(WJ) and continuous EV harvesting with scalable purification. It achieves high cell expansion, preserves MSC identity and viability, continuously produces large amounts of high-purity EVs, and confirms EV uptake by relevant target cells.
With a particle yield factor of approximately 1.2 × 10^4 EVs per cell per day, this approach provides sufficient material for clinical doses and sets the stage for more standardised, cost-effective MSC-EV therapeutics.
Reference:
Ulpiano C., Salvador W., Franchi-Mendes T., et al. Continuous collection of human mesenchymal-stromal-cell-derived extracellular vesicles from a stirred tank reactor operated under xenogeneic-free conditions for therapeutic applications. Stem Cell Research & Therapy. 2025;16:210. https://doi.org/10.1186/s13287-025-04341-2
Interested in microcarriers for your cell culture?
We offer high-quality dissolvable microcarriers optimised for efficient MSC attachment and expansion in bioreactor systems. Learn more on our product page or contact us at info@smartmcs.com.au.
Disclaimer: The study referenced in this blog did not involve Smart MCs microcarriers. It is shared here to provide context on current research in the area of microcarrier-based cell culture.




Leave A Comment