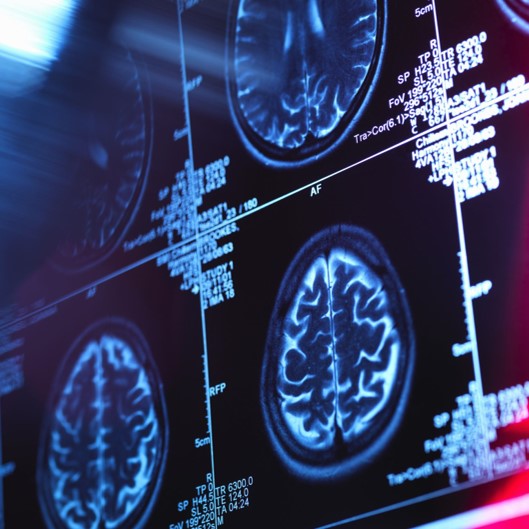
An article recently published by GEN describes the unique benefits of stem cells when treating Multiple Sclerosis. Such discoveries support a growing demand for stem cell therapies and clinical applications of such techniques in the future. Supporting this requirement calls for higher adherent quantities of stem cells in clinical settings, therefore microcarriers are predicted to play a broadened role for achieving higher scaled production. See Original Article.
The article written by Sue Pearson discusses a clinical trial conducted by Dame Pamela Shaw, MD professor of neurology and director of the Sheffield Institute for Translational Neuroscience (SITraN). The trial measured the effectiveness of Autologous Hematopoietic Stem Cell Transplantation (AHSCT) as a potential treatment for Multiple Sclerosis (MS). This remedy involves an intensive chemotherapy treatment aimed at ‘resetting’ the immune system through regrowing it partially or fully, through usage of stem cells in order to prevent it further attacking the body. Neurologists acknowledge the significant unmet medical need in MS treatment, indicating AHSCT could present as a promising transformative treatment. However further research is needed to demonstrate the safety and value of cell therapies.
Data from the European Society for Blood and Marrow Transplantation (EBMT) registry supports the efficacy and low incidence of adverse events with AHSCT. Joachim Burman, PhD, adjunct senior lecturer at the Department of Medical Sciences, who has also performed open trials of AHSCT, stated it has a positive effect on brain health and after four years following the trial, neuronal damage had been completely halted in patients from treatment of AHSCT.
Despite the evidence, the accessibility and acceptance of AHSCT varies across countries, often offered as a third-line therapy due to scepticism and limited awareness among neurologists. The importance of prioritising effective treatments earlier in the disease progression and ongoing clinical trials such as the StarMS trial is highlighted, which aims to generate more evidence to support AHSCT as a first or second-line therapy for severe MS patients.
Giving relevance to his article, AHSCT is one of a vast array of applications in which stem cell therapy is utilised. Adherent cell production is vital for treating other life-threatening illnesses. One notable method of scaling up production of such stem cells is through bioreactor-based cell culture. While multi-layer flasks and cell factories improve surface area-to-volume ratio, they lack scalability. Bioreactors offer efficient automation through utilisation of microcarriers enabling the growth of such cells in suspension. This solution provides huge potential for producing stem cells at scale, fuelling the vast array of disease-treating applications stem cells hold.
To learn more, visit our knowledge centre.
Original Article: Pearson S. (March, 2023) Stem Cells May Move to the Front Line against Multiple Sclerosis, GEN.




Leave A Comment