Adeno-associated virus (AAV) vectors are at the forefront of gene therapy, offering great potential for treating a variety of genetic disorders. However, a significant challenge in the field is the limited production capacity of these vectors. Higher specific yields of AAV vectors are often achieved using adherent cell systems compared to suspension cultures, and microcarrier-based culture systems have emerged as a viable solution for large-scale production of anchored cells.
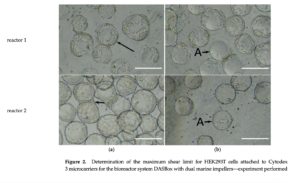
A recent study has explored the potential of microcarrier cultures to address the production bottleneck of AAV vectors. This investigation focused on the triple plasmid transfection of HEK293T cells in a stirred tank bioreactor, providing a proof-of-concept for a continuous AAV vector production process. Initially, HEK293T cells were grown and expanded in suspension, taking advantage of the operational ease of this method. The cells were then anchored on microcarriers for plasmid transfection, facilitating transient AAV vector production in a 200 mL stirred-tank bioreactor.
The study highlighted the impact of shear stress on cell viability and vector yield, crucial factors for optimizing bioreactor conditions. Moreover, the feasibility of a continuous production process was evaluated, demonstrating that continuous transfection and inoculation of microcarriers can enhance production capacity without increasing the reactor’s footprint.
Gene therapy is poised to revolutionize medicine, with AAV vectors being a leading candidate. However, scaling up production to meet the demands of extensive clinical trials and eventual commercialization remains a challenge. Traditional 2D culture methods, while effective at the discovery phase, are not scalable enough for large-scale production. Microcarrier-based cultures offer a scalable solution, enabling the adaptation of existing transfection protocols without extensive optimization.
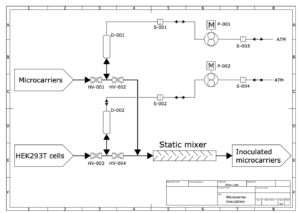
The present study demonstrated that a 2D transfection process using T-flasks can be successfully scaled up to a 200 mL bioreactor with an effective surface area of over 1600 cm². Importantly, the study provided proof-of-concept for a continuous transfection process, which could significantly increase production capacity while maintaining a compact reactor footprint.
A fully automated continuous transfection process could reduce the variability associated with manual transfections, allowing for greater control over key parameters such as DNA and PEI concentrations. This level of control could optimize transfection efficiency and provide insights into the effects of different medium components on AAV production. Unlike batch processes, which suffer from heterogeneity due to localized mixing, continuous processes ensure a uniform distribution of cells on microcarriers and consistent transfection conditions.
Scaling up to a 2000 L reactor in batch mode presents significant challenges, such as ensuring homogenous mixing of large volumes of PEI-DNA complexes. Continuous transfection offers a solution to this problem, providing a more controlled and efficient process for large-scale AAV vector production.

In conclusion, the study presents a promising approach for enhancing AAV vector production using microcarrier-based cultures in stirred tank bioreactors. This advancement not only addresses current production limitations but also paves the way for scalable and efficient gene therapy manufacturing processes.
For more information on our innovative solutions and to stay updated on the latest developments, visit our website or contact our team at Smart MCs.




Leave A Comment