How to Minimise Unwanted Cell Attachment to Non-Microcarrier Surfaces in Microcarrier Culture
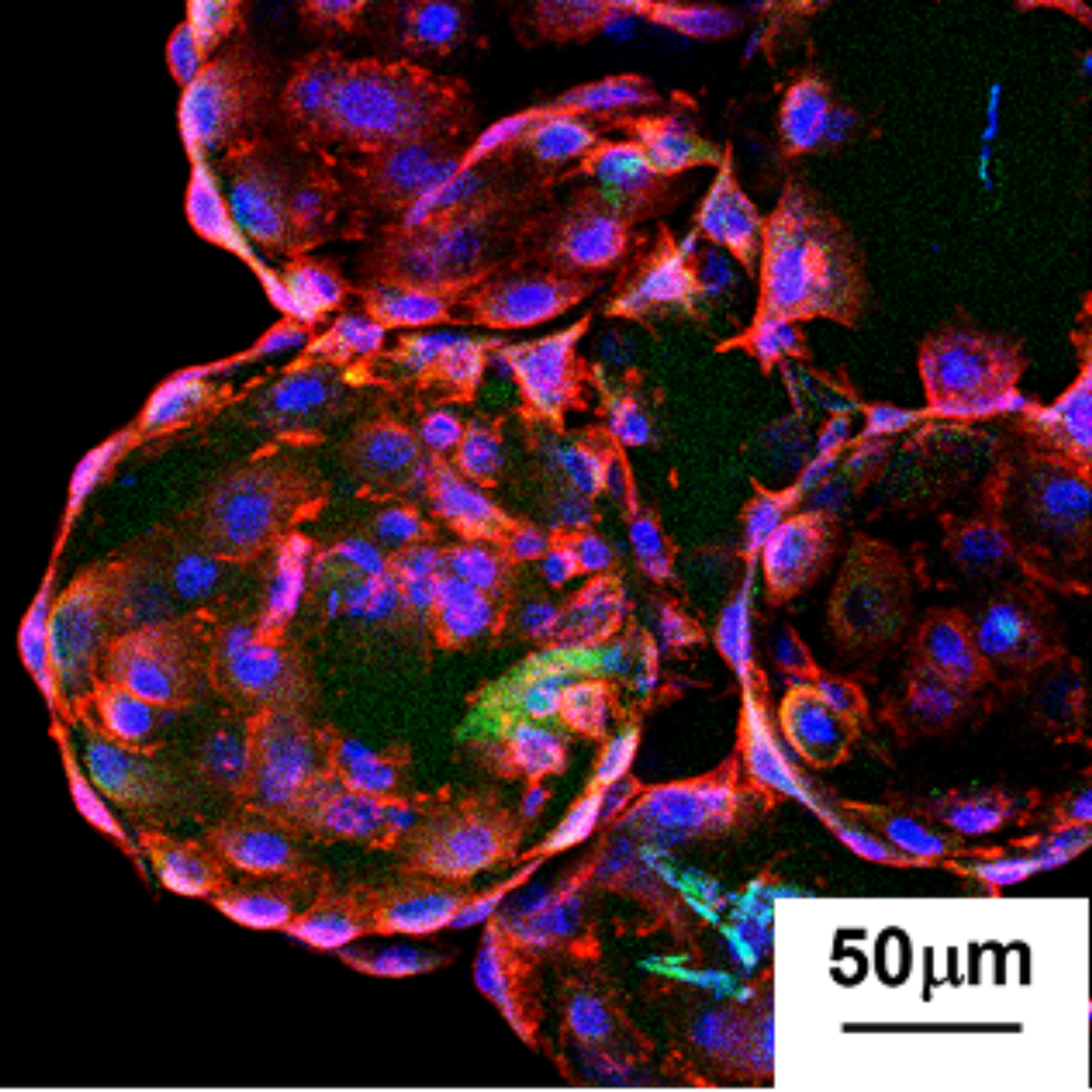
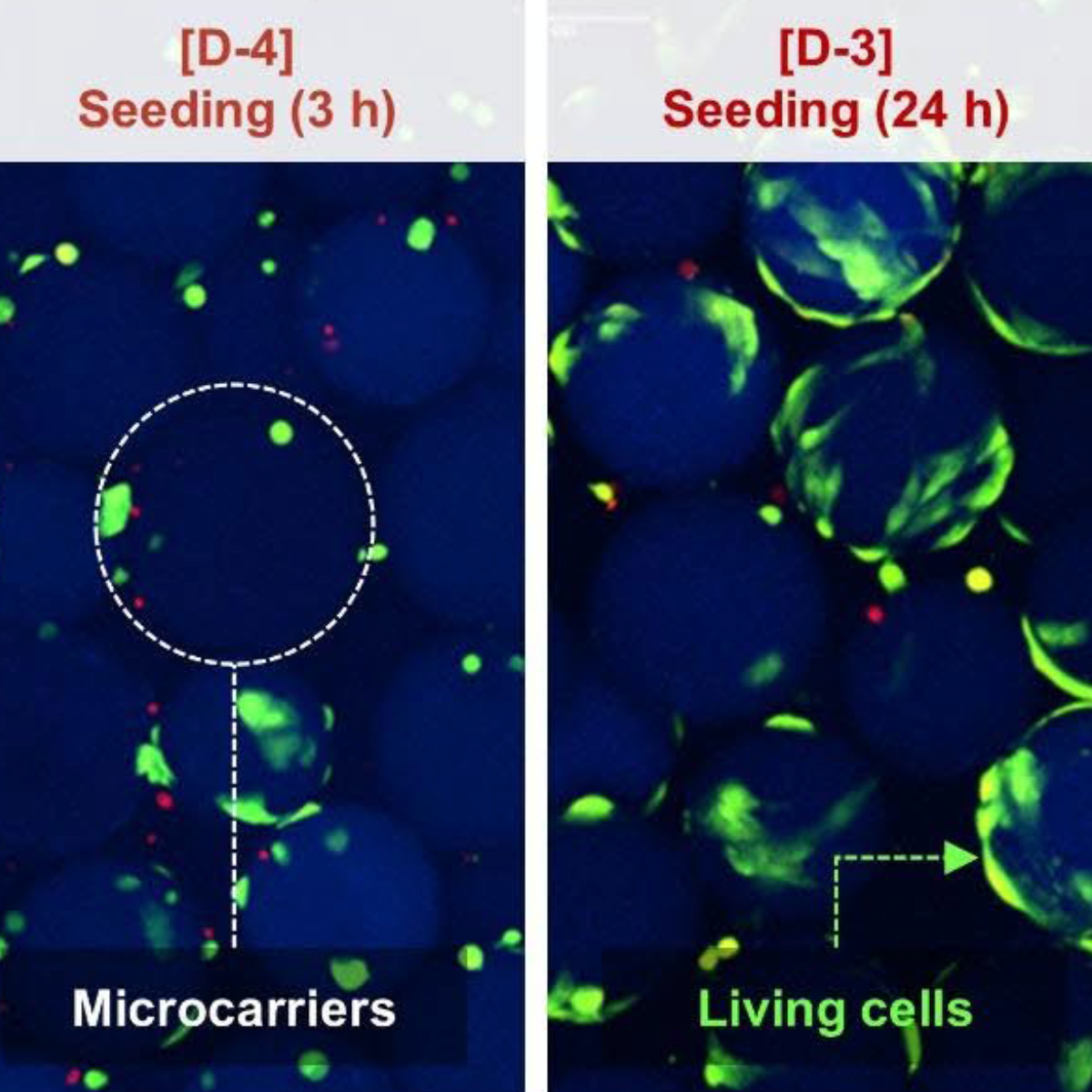
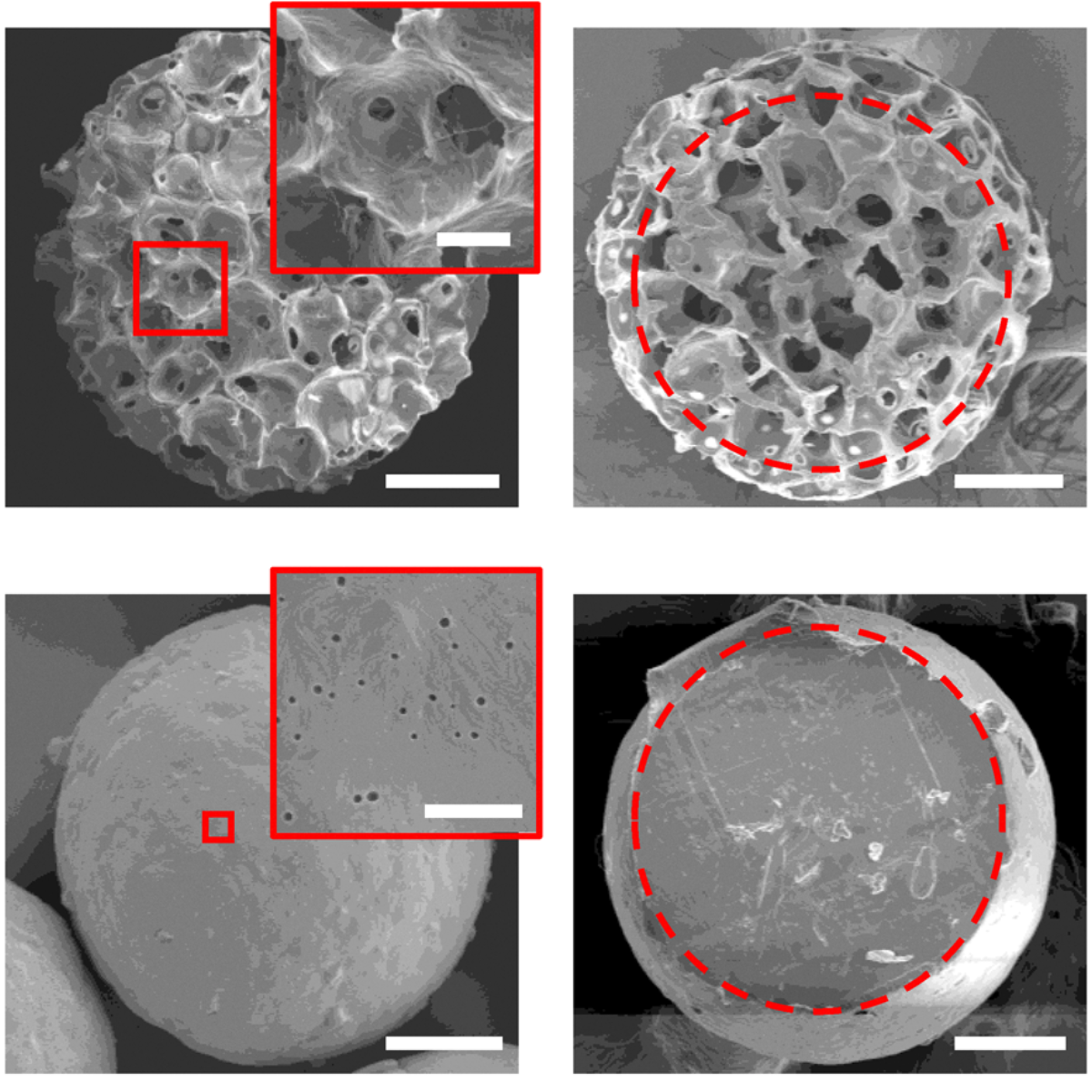
“Why are my cells sticking to the vessel when I’m using microcarriers?” It’s one of the most common questions researchers ask when working with microcarrier-based cell culture, especially with highly adherent cells like mesenchymal stromal cells (MSCs). You’ve added microcarriers. Your cells are viable. Agitation looks reasonable. Yet after 24 hours, cells are clearly [...]