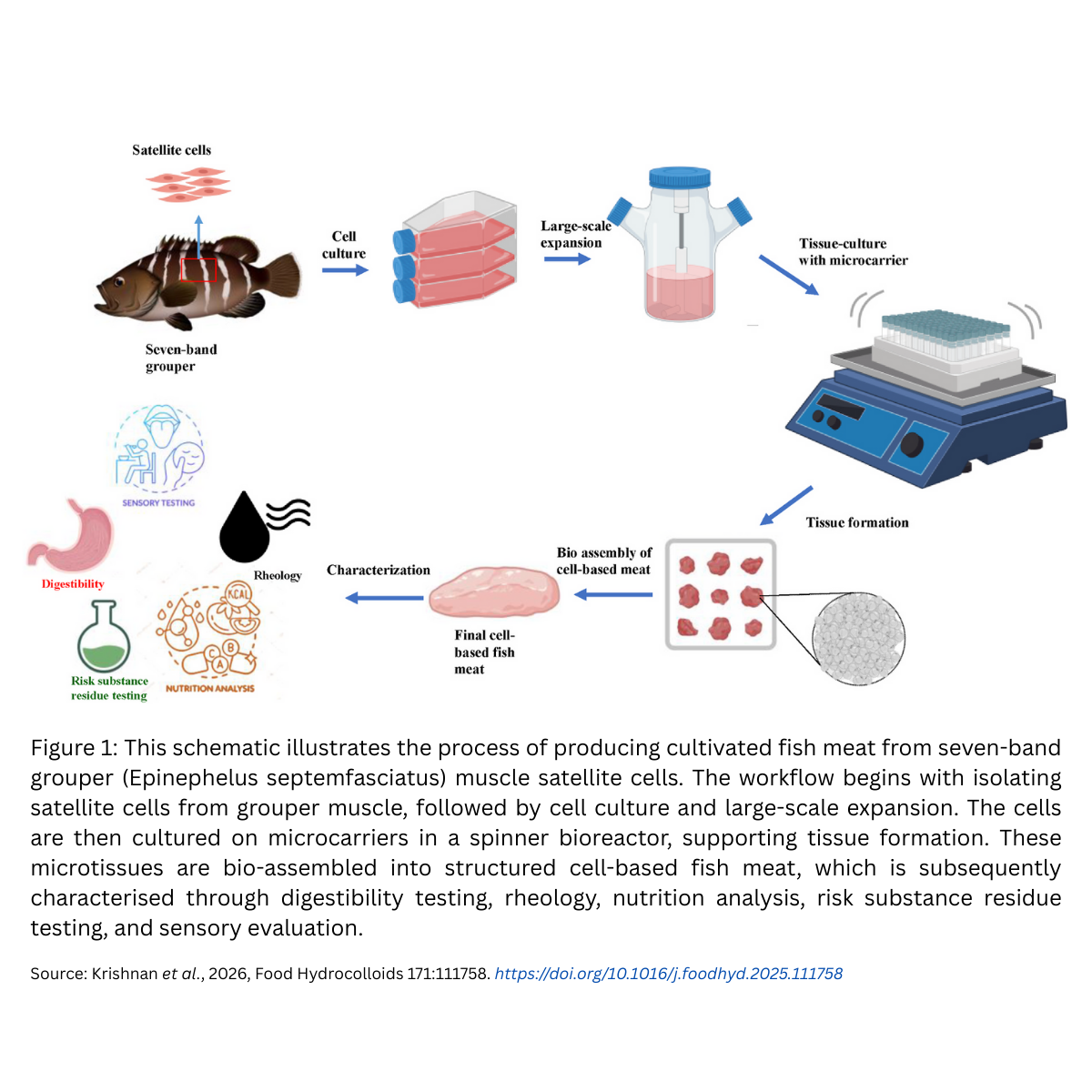
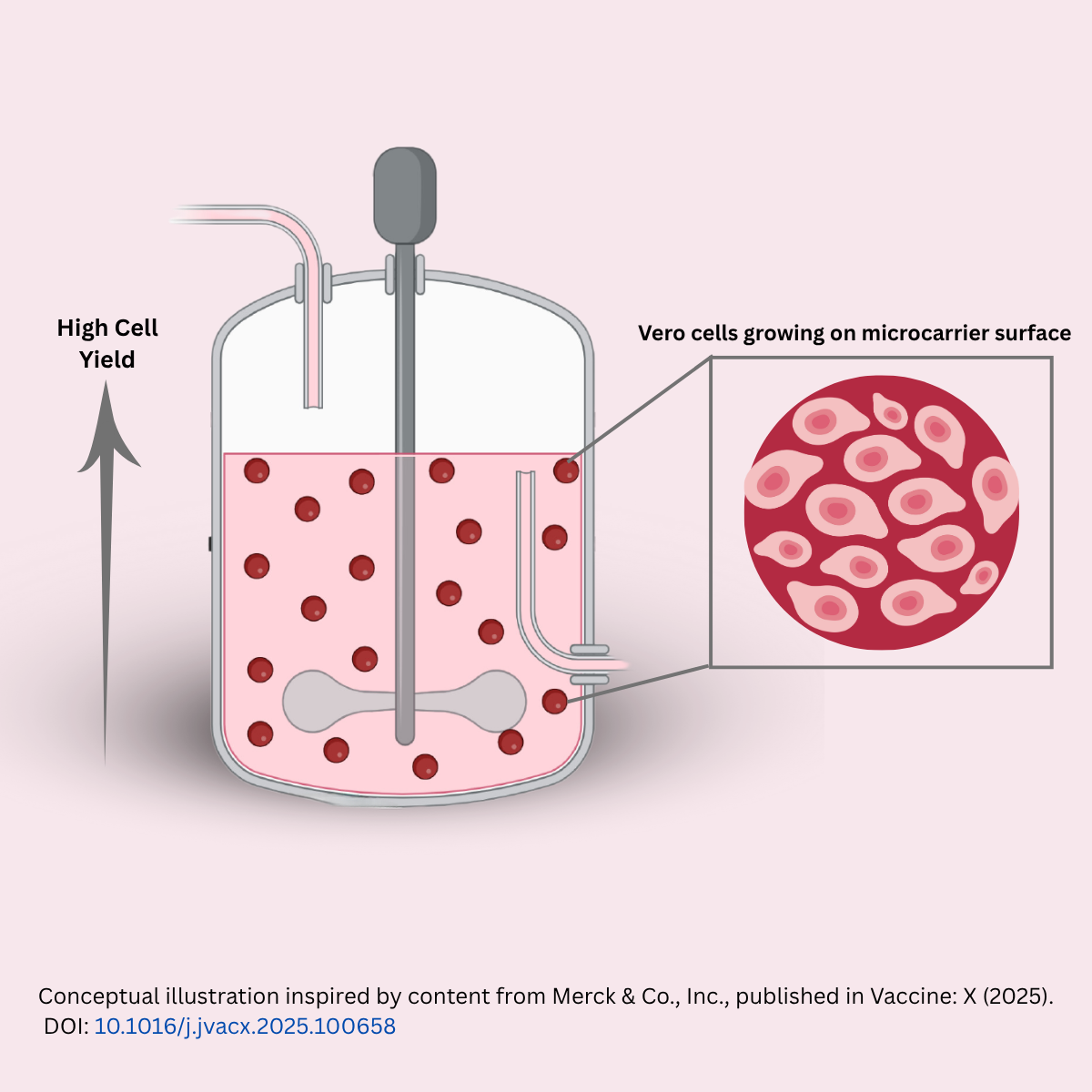
September’2025 – Scientific Webinar: Prof. Kristopher Kilian
We were pleased to host Prof. Kristopher Kilian from UNSW Sydney for our September webinar, where he presented on: Inspired by Nature: Biomaterials Design for Therapeutic Cell Production and Delivery Presentation Highlights Supramolecular, self-healing hydrogels: Prof. Kilian presented Trpzip hydrogels with tuneable mechanics, low yield point, shear-thinning, and self-healing behaviour, along with stress relaxation similar [...]