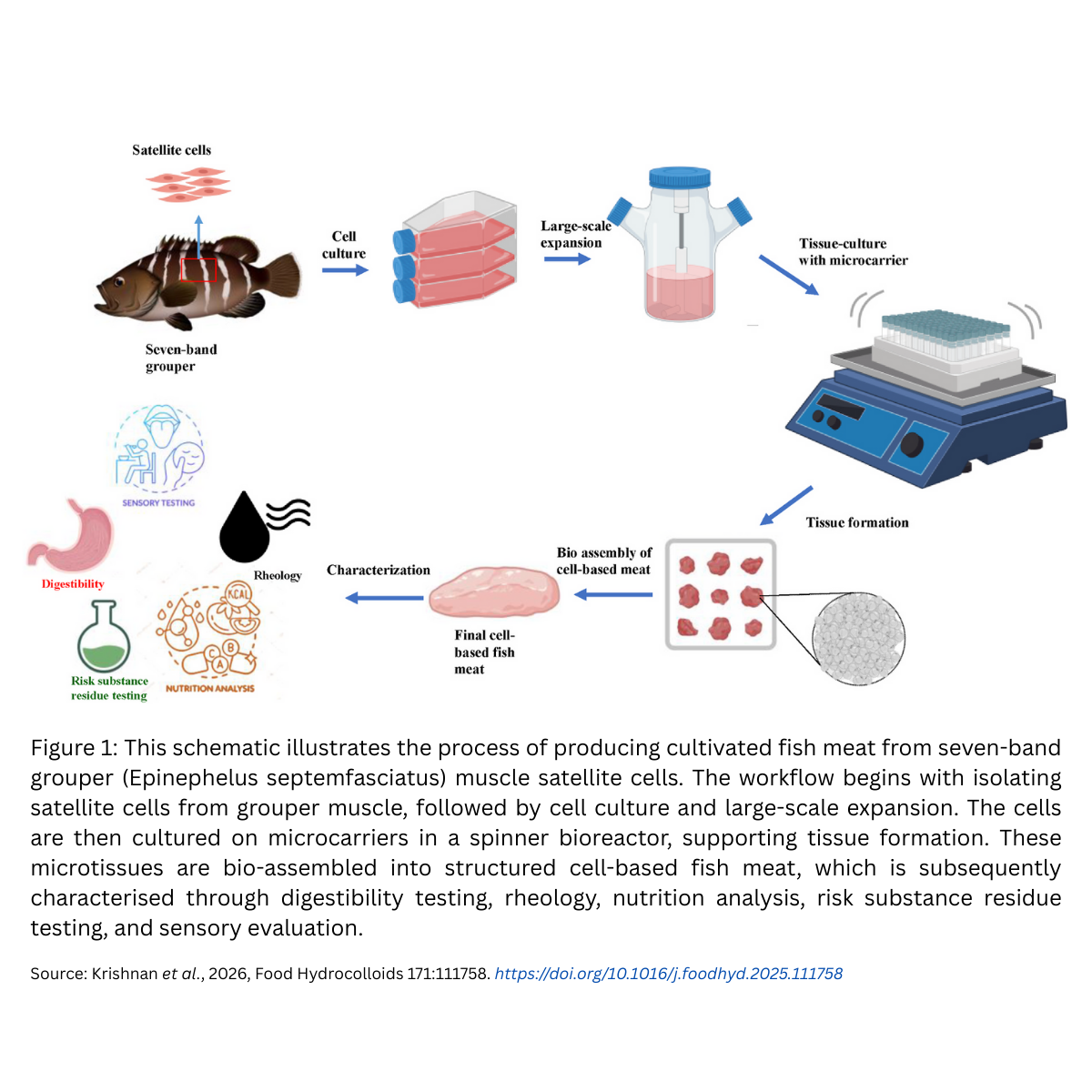
The global demand for seafood continues to climb. Yet overfishing, habitat destruction, and pollution from heavy metals and microplastics make traditional fishing and aquaculture less sustainable every year. One of the most exciting solutions to this challenge is cell-based fish meat, grown directly from fish cells in bioreactors, without needing to raise or harvest whole fish.
While cultivated beef, pork, and chicken are already making their way into commercial products, fish has lagged behind. That’s why a new study from Pukyong National University, South Korea, is so important. It demonstrates a working pathway to cultivated fish meat and, most importantly for us in biomaterials, highlights the central role of microcarriers in making large-scale cell culture possible.
Inside the Study
The paper, published in Food Hydrocolloids (2026), is titled:
Here’s what the researchers did:
- Cells used – muscle satellite cellsMuscle satellite cells are a special type of stem-like cell found in muscle. In a living fish, they “wake up” when muscle is damaged or growing, repairing and building new muscle fibres. Because they can both multiply quickly and differentiate into mature muscle tissue, they are the perfect cell type for cultivated meat. This study used satellite cells from the seven-band grouper, a premium food fish in Asia.
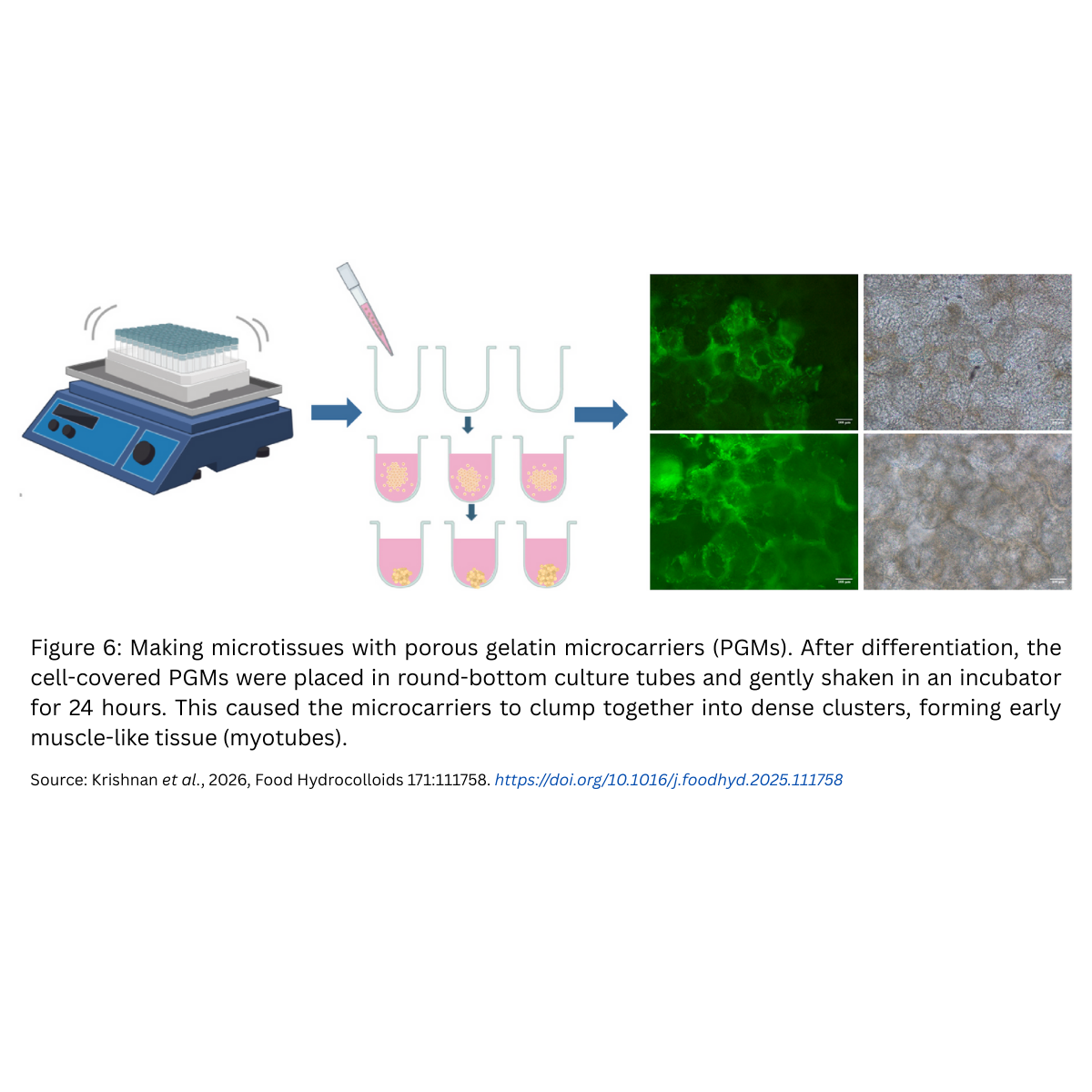
- Microcarriers used – porous gelatin microcarriers (PGMs)The cells need somewhere to sit and grow, otherwise they float around in liquid without anchoring. This is where microcarriers come in. Think of them as tiny edible sponges made of gelatin. Each microcarrier has thousands of holes (pores) that give cells plenty of surface area to attach, spread, and multiply. Because they are edible, they can stay in the final product.In this study, the researchers used porous gelatin microcarriers (PGMs) as edible scaffolds.†
- Culture system – spinner flask bioreactorThe cells and microcarriers were placed into a spinner flask bioreactor, essentially a flask with a magnetic stirrer that gently moves the mixture. This keeps the microcarriers evenly suspended, ensuring all cells receive nutrients and oxygen while preventing clumping.
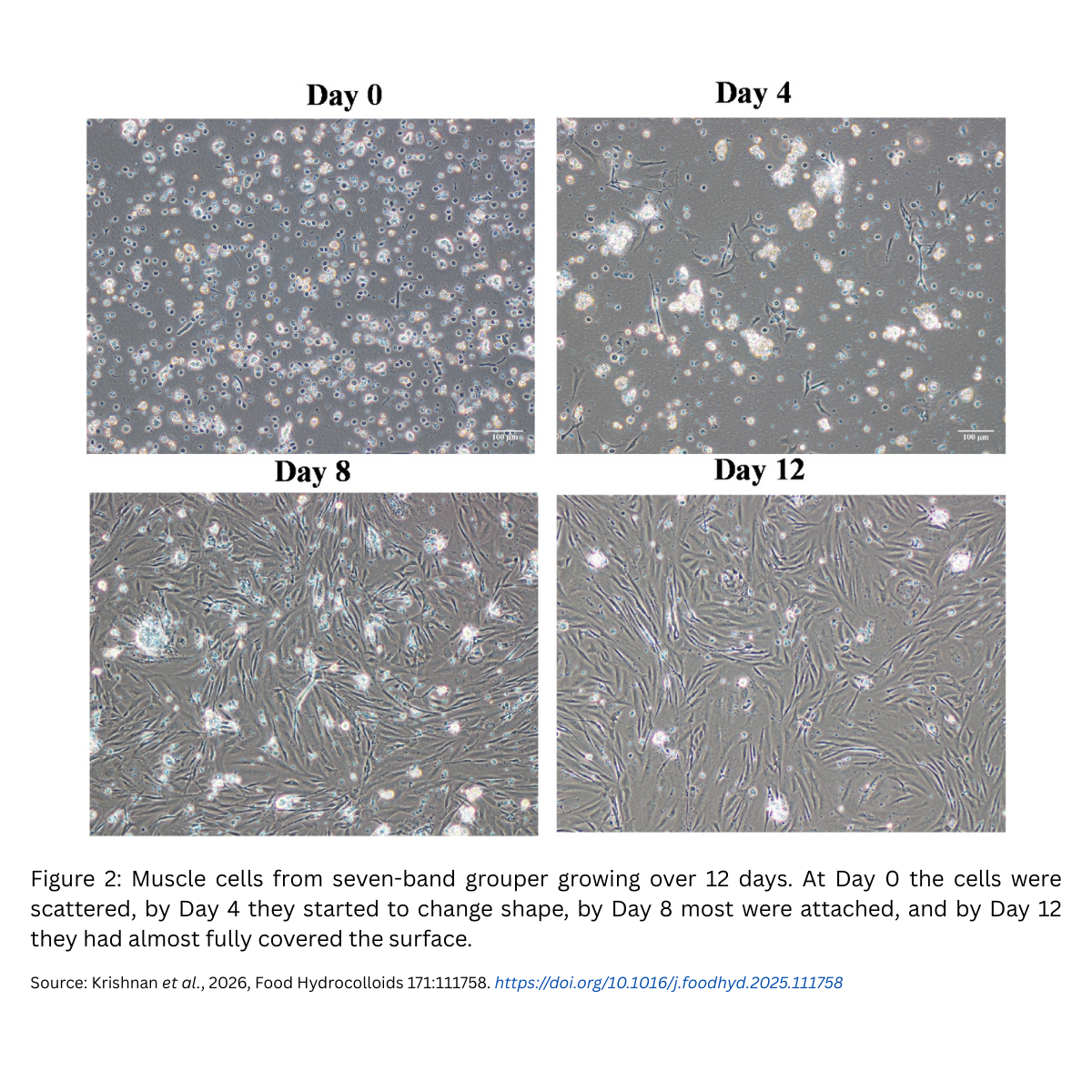
- Scale achieved – sevenfold increase in nine daysStarting with a certain number of cells, the team achieved a sevenfold increase after nine days of culture. This shows that the microcarriers created a healthy environment for rapid cell expansion.
- Differentiation – turning into muscle tissueMultiplication alone isn’t enough. The cells also need to become actual muscle fibres. The researchers confirmed this by detecting specific protein markers:
- Pax7 and MyoD → indicate active cell growth and preparation for muscle formation.
- MyoG, Desmin, and MHC → show that cells have successfully differentiated into mature muscle.
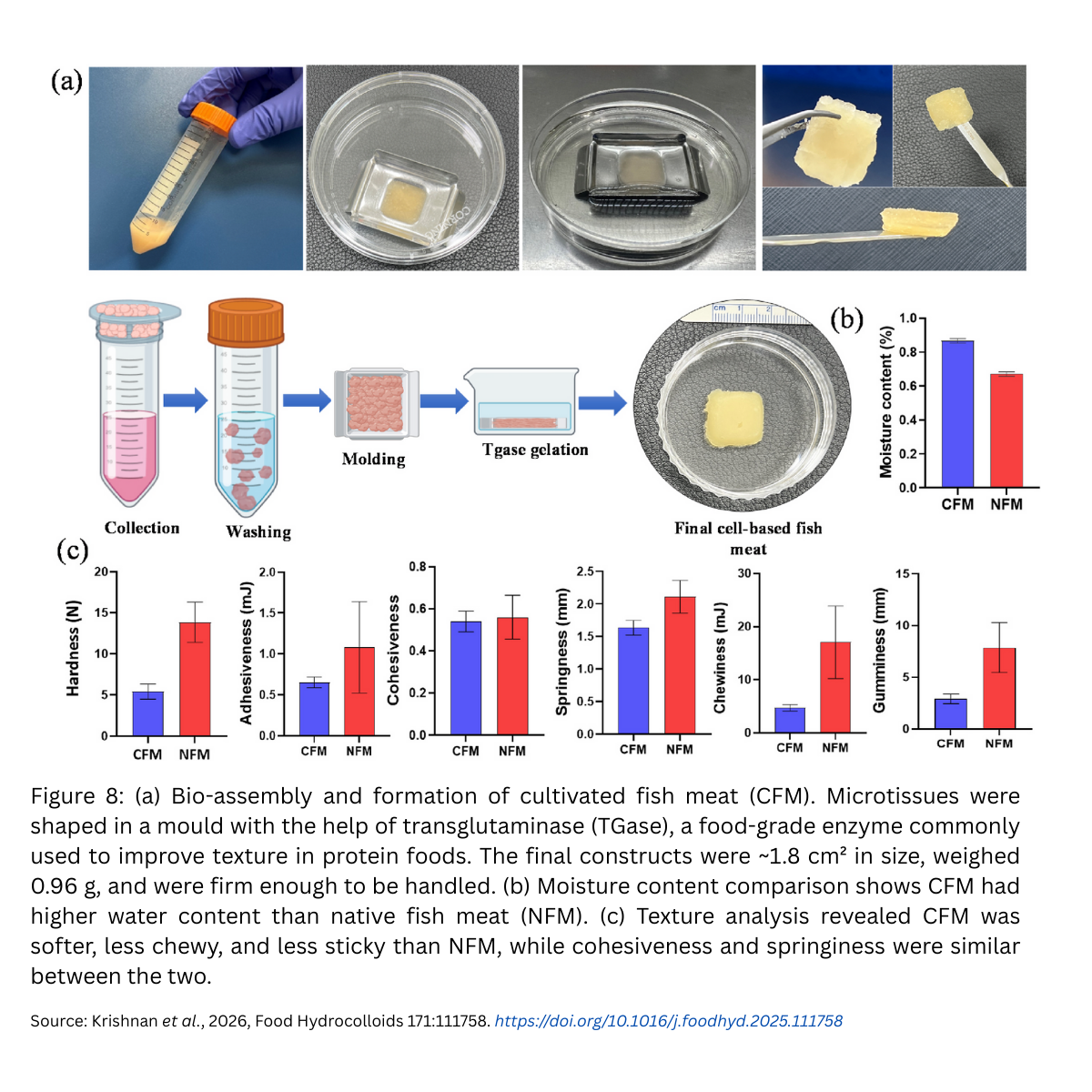
- Assembly – creating the fish meatAfter cells grew and matured, the microcarriers naturally clumped together into microtissues (tiny clusters of muscle). These were then “glued” together using transglutaminase (TGase), a safe enzyme widely used in food processing to improve texture. The result was structured pieces of cultivated fish meat (CFM).
Results at a Glance
- Cell growth: Cells expanded sevenfold in nine days on the microcarriers.
- Muscle formation: Cells expressed the correct muscle proteins, proving they matured properly.
- Texture: Cultivated fish meat was softer and less chewy than natural fish, but still cohesive and springy.
- Moisture: CFM contained more water (79.5%) compared to natural fish (71.3%).Digestibility: In simulated digestion, protein digestibility was higher in CFM (88.3%) compared to native fish (81.6%).
Flavour and Taste Results
The researchers didn’t stop at cell growth, they also asked the question every consumer will eventually ask: how does it taste?
- Higher umami (7.8 vs 4.2 for natural fish)
- Higher saltiness (7.1 vs 4.9)Slightly lower sournessThe stronger umami profile was linked to higher levels of free amino acids like glutamic acid and aspartic acid, which naturally enhance savoury taste.
- CFM contained more pleasant aroma molecules like propenol (sweet, fatty notes) and 2-propanol (floral).
- Natural fish contained more aldehydes such as hexanal, responsible for “fishy” or rancid odours.
- Overall, CFM had a cleaner, sweeter flavour profile.
Why This Matters for Microcarriers
This study highlights how essential microcarriers can be for cultivated meat production.
The edible microcarriers used by the researchers demonstrated strong cell attachment, rapid expansion, and successful differentiation into muscle tissue. This is an important validation of how scaffolds can directly support both the growth process and the quality of the final product.
As the industry moves toward larger scales and a wider range of species, there is also growing interest in microcarriers that can be tailored for specific applications. This is where the field is heading, and where next-generation customisable microcarriers will play an important role in enabling flexibility and scalability.
While this particular study used materials selected by the research team, it underscores the vital role microcarriers play in cultivated meat.
Smart Microcarriers
At Smart MCs, we’re not just another supplier, we’re building the future of biomaterials.
Our customisable microcarriers are designed to build on the progress already demonstrated in the field and give researchers more control over key features:
- Customisable design: Porosity, stiffness, and surface chemistry can be adjusted to suit your specific cell type (fish, poultry, mammalian, or stem cells).
- Cross-species compatibility: One platform can support multiple cell types, enabling versatility across cultivated seafood and meat projects.
- Bioreactor-ready formats: Optimised for industrial-scale suspension culture.
- Food-grade materials, with development pathways toward GMP compliance for future commercial needs: Supporting research today and preparing for future commercial production standards.
Where recent studies highlight what’s possible with edible microcarriers, Smart MCs is focused on advancing the next generation of microcarriers, supporting researchers and companies as they work to scale, fine-tune performance, and bring cultivated meat closer to mainstream adoption.
Final Thoughts
This landmark study from Pukyong National University proves that cultivated fish meat is not only possible but promising. From stronger umami flavour to improved digestibility, the results highlight the potential of cell-based seafood to deliver a sustainable, tasty, and nutritious alternative.
The research also reinforces what we at Smart MCs know well: microcarriers are a key enabler of scalable cultivated meat. And as the field continues to evolve, next-generation solutions will be central to accelerating progress.
👉 That’s where Smart MCs comes in.
- Find out more about our customisable microcarriers
- Discover how to source microcarriers tailored to your unique cells and processes
- ✨ Smart MCs: smarter scaffolds, better cells, sustainable food.
† Clarity note: This post summarises independent, published research. The microcarriers used in the study were not Smart MCs products.
Reference:
Krishnan, S., Ulagesan, S., Choi, Y-H., & Nam, T-J. (2026). Scalable production of cell-based fish meat constructs using 3D porous gelatin microcarriers and seven-band grouper (Epinephelus septemfasciatus) muscle satellite cells. Food Hydrocolloids, 171, 111758. https://doi.org/10.1016/j.foodhyd.2025.111758




Leave A Comment