Microcarrier Innovations: Paving the Way for Large-Scale Mesenchymal Stromal Cell Expansion
A recent review published in the Journal of Trends in Biotechnology highlights the critical advancements and challenges in the field of mesenchymal stromal cell (MSC) expansion through microcarrier technology (Read More – DOI: 10.1016/j.tibtech.2024.01.001).
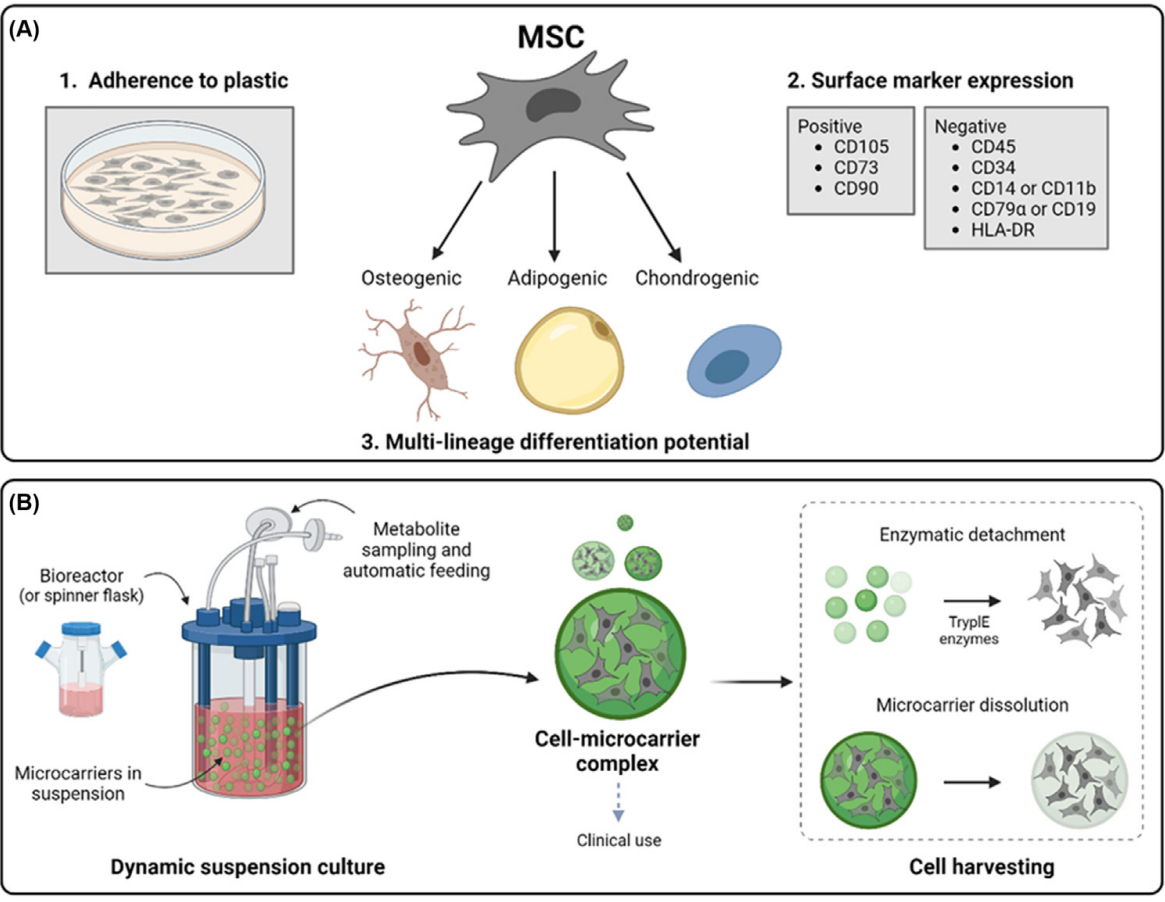 With advancements in cellular therapy, the crucial need for large-scale expansion of mesenchymal stromal cells (MSCs) is clearer than ever. This expansion is vital for the practical application of MSC-based clinical therapies, which hold the promise of treating a wide range of medical conditions. At the forefront of this scientific breakthrough are microcarrier expansion systems, which are set to revolutionize the way we produce these therapeutic cells, making the process both cost-effective and efficient.
With advancements in cellular therapy, the crucial need for large-scale expansion of mesenchymal stromal cells (MSCs) is clearer than ever. This expansion is vital for the practical application of MSC-based clinical therapies, which hold the promise of treating a wide range of medical conditions. At the forefront of this scientific breakthrough are microcarrier expansion systems, which are set to revolutionize the way we produce these therapeutic cells, making the process both cost-effective and efficient.
The Challenge with Current Methods
The traditional methods of expanding MSCs for clinical use have been fraught with challenges. Primarily, the use of commercial polystyrene microcarriers has been problematic, plagued by issues of poor reproducibility and efficiency. This inefficiency hampers the production of the necessary quantities of therapeutic cells, impacting the feasibility of MSC-based treatments.
A New Era of Microcarriers
In response to these challenges, a new generation of microcarriers has emerged. These innovative carriers are tuneable, meaning their physical, mechanical, and biological properties can be precisely adjusted to create the ideal environment for MSC growth and proliferation. This adaptability marks a significant improvement in cell expansion outcomes, promising to enhance the quality and quantity of MSCs available for therapy.
These advanced microcarriers demonstrate their effectiveness in both static and dynamic culture conditions, crucially without compromising the inherent stemness and differentiation potential of the MSCs. This ensures that the expanded cells maintain their therapeutic potential.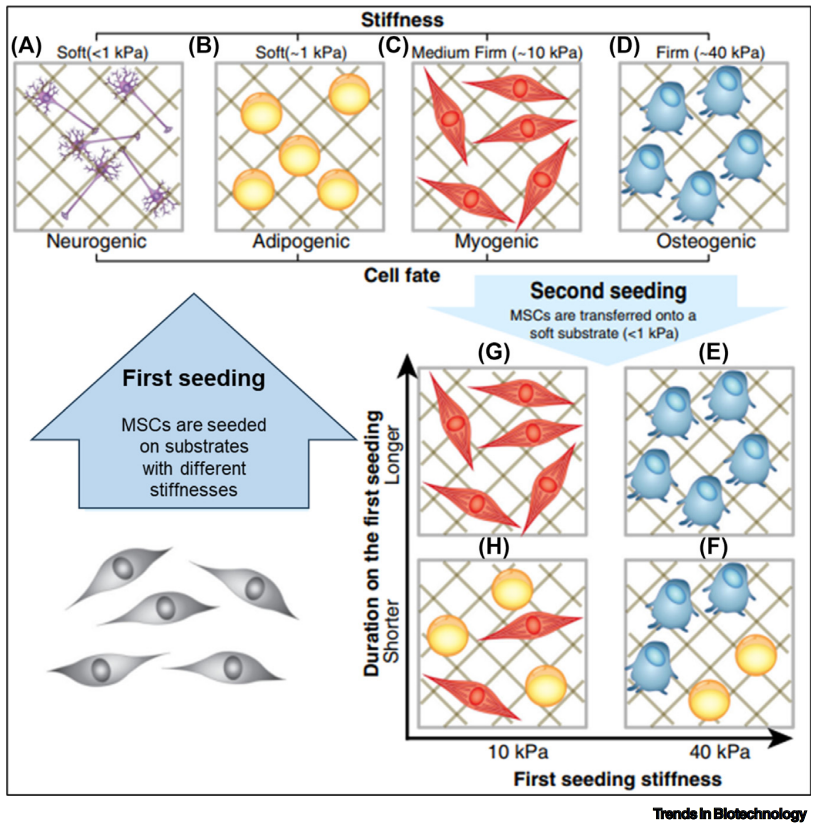
Scaling Up with Dynamic Bioreactors
The integration of these tuneable microcarriers with dynamic bioreactors represents a leap forward in the scalability of MSC expansion. This combination facilitates the efficient expansion and harvesting of MSCs, a critical step towards transitioning laboratory achievements into actual clinical applications that can benefit a broad patient base.
The Path Forward: GMP-Grade Systems and Beyond
The path to fully leveraging these technological advancements involves the development of good manufacturing practice (GMP)-grade systems. Such systems, along with the adoption of nonxenogenic culture media and the optimization of bioreactors tailored to the novel microcarriers, are indispensable for improving cell yield and ensuring reproducibility. These improvements are key to producing MSC-based therapies on a scale that meets clinical demands, thereby making these treatments more accessible and reliable for patients worldwide.
Conclusion
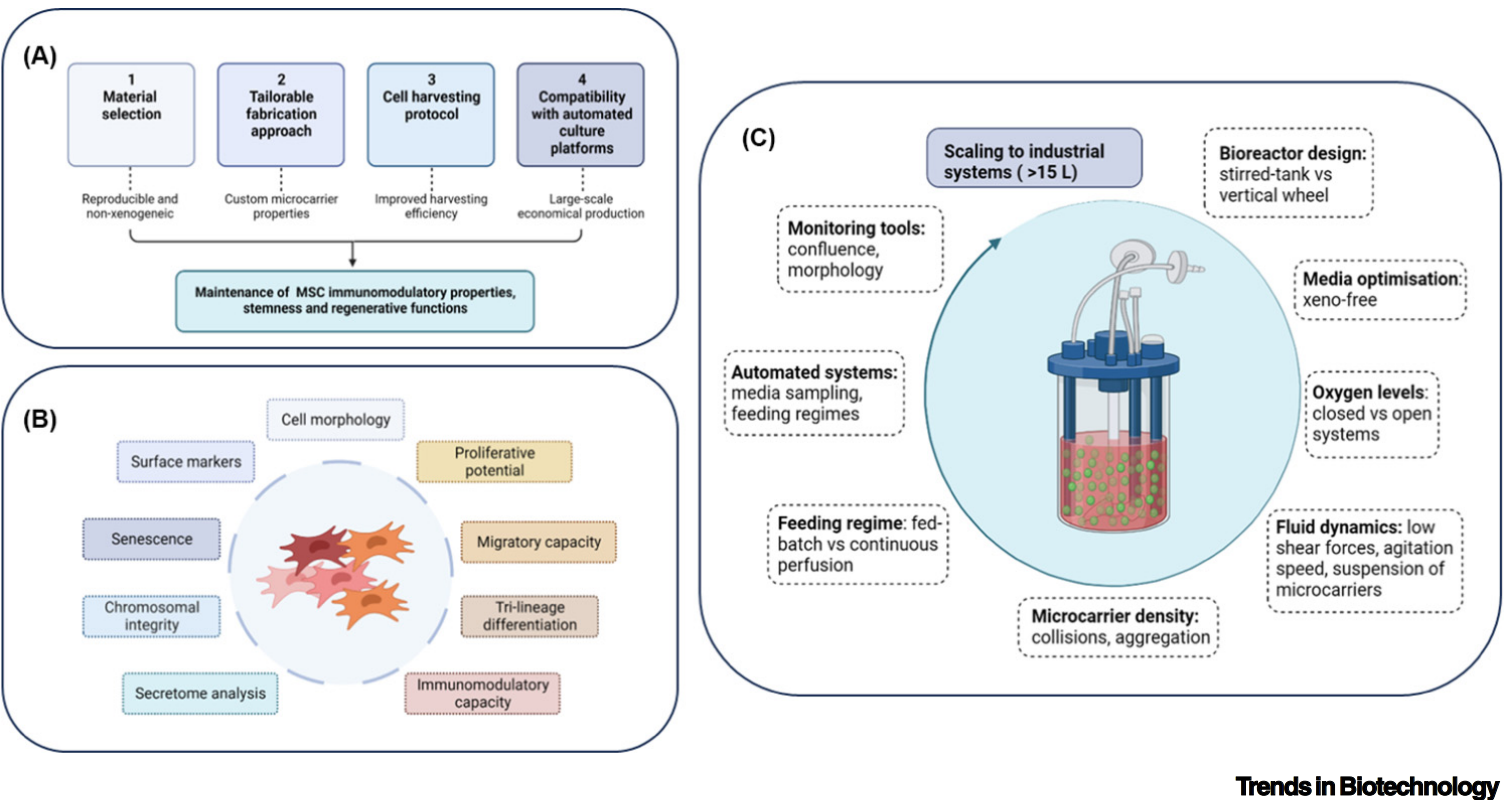
The advancement of microcarrier technology stands at the heart of the future of MSC-based therapies. By overcoming the current limitations and utilizing dynamic culture systems, we can greatly enhance the process of MSC expansion. This progress not only promises to make clinical therapies more efficient and effective but also opens the door to bringing life-changing treatments to patients globally. The evolution of microcarriers is indeed setting the stage for a new chapter in regenerative medicine.




Leave A Comment