A recent publication titled “Scalable manufacturing of gene-modified human mesenchymal stromal cells with microcarriers in spinner flasks” in the Journal of Biotechnological Products and Process Engineering addresses a critical challenge in gene-modified cell therapy: developing scalable manufacturing methods for clinical applications.
Human mesenchymal stromal cells (hMSCs) are known for their immunomodulatory properties and in vitro differentiation ability, leading to their investigation in over 1000 clinical trials in the past decade. With many studies on gene-modified hMSC-based products advancing to early clinical trials, there is a significant need for efficient manufacturing processes to produce sufficient doses of these cells.
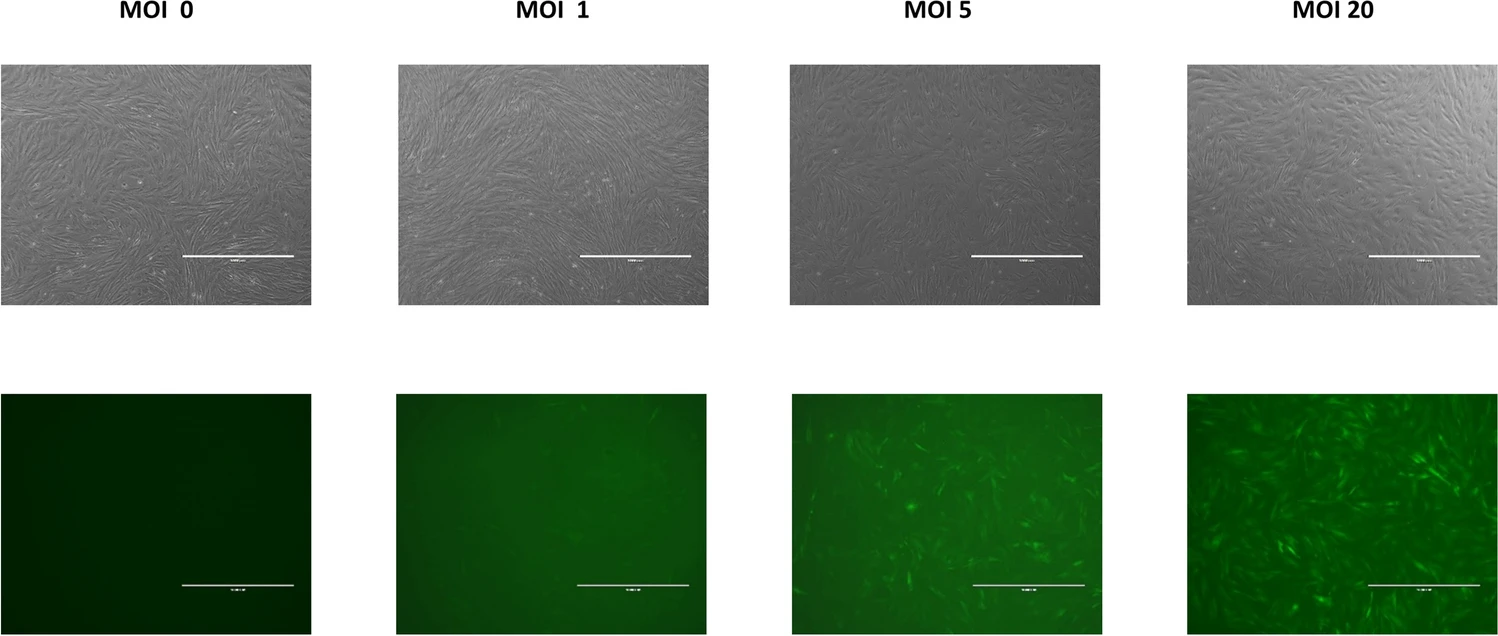 This study demonstrates a scalable manufacturing process using a microcarrier-bioreactor system for the expansion of gene-modified hMSCs. Umbilical cord tissue mesenchymal stromal cells (UCT-hMSCs) were transduced with lentiviral vectors carrying either green fluorescent protein (GFP) or vascular endothelial growth factor (VEGF) transgenes. These cells were then cultured in 100 mL spinner flasks with Spherecol microcarriers and expanded over seven days. Both non-transduced and transduced cells achieved comparable maximum cell concentrations (≈1.8 × 10^5 cell/mL) after six days of culture.
This study demonstrates a scalable manufacturing process using a microcarrier-bioreactor system for the expansion of gene-modified hMSCs. Umbilical cord tissue mesenchymal stromal cells (UCT-hMSCs) were transduced with lentiviral vectors carrying either green fluorescent protein (GFP) or vascular endothelial growth factor (VEGF) transgenes. These cells were then cultured in 100 mL spinner flasks with Spherecol microcarriers and expanded over seven days. Both non-transduced and transduced cells achieved comparable maximum cell concentrations (≈1.8 × 10^5 cell/mL) after six days of culture.
Key findings include:
- Successful transduction of hMSCs with GFP and VEGF transgenes using lentiviral vectors.
- Efficient expansion of transduced hMSCs on microcarriers in spinner flasks over seven days.
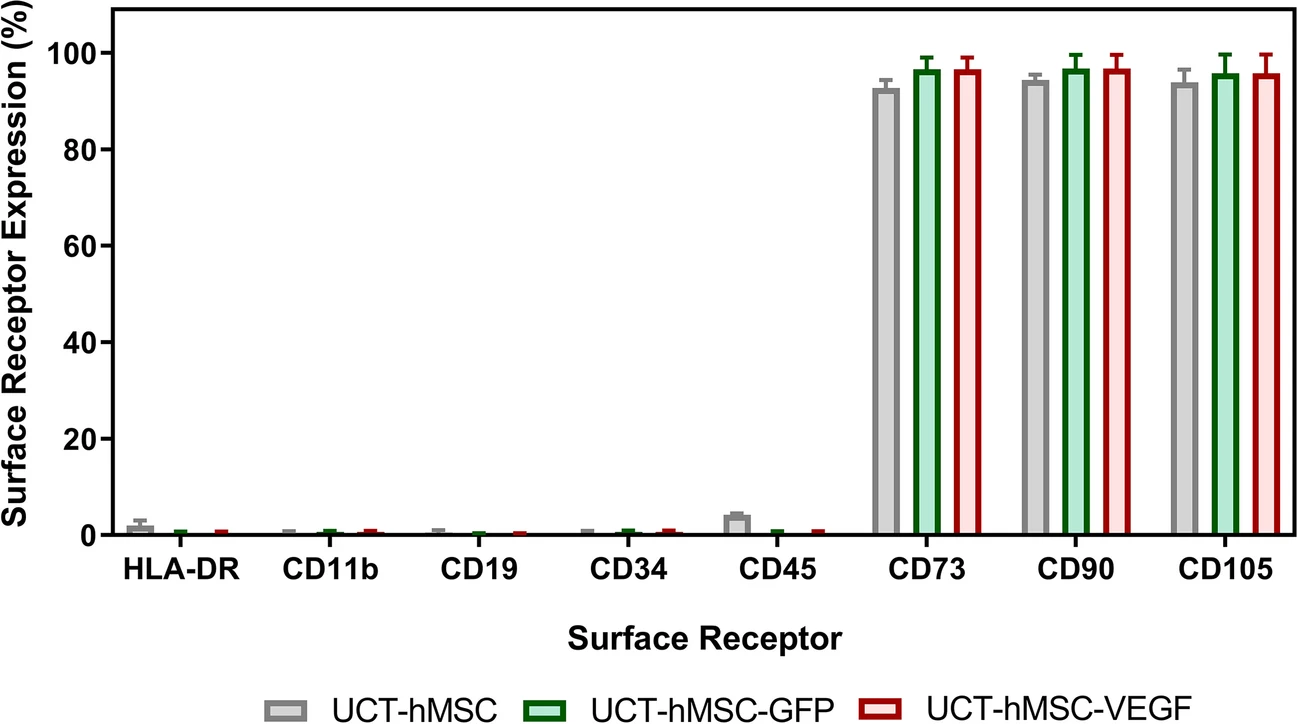
- Genetic modification did not negatively impact the immunophenotype characteristics of the hMSCs, maintaining high levels of CD73, CD90, and CD105 expression (>90%) and low levels of negative markers (CD11b, CD19, CD34, CD45, and HLA-DR) (<5%).
These results align with the criteria established for hMSCs by the International Society for Cell and Gene Therapy (ISCT), highlighting the potential of this scalable manufacturing process to overcome significant translational and commercial bottlenecks in gene-modified cell therapy.
Microcarriers are essential for large-scale production of MSCs, enabling efficient cell expansion while maintaining cell quality and characteristics.
Smart MCs is at the forefront of providing the next generation of microcarriers that can be customized and tailored for various applications. Please get in touch with our experts if you are looking to scale up your culture and advance your cell therapy products.





Leave A Comment