Check out this publication on how scaling bovine viral vaccine production can be enabled using Microcarriers:
Vaccination remains the most effective method for controlling and preventing diseases, ensuring the safe trade of live animals. The manufacturing of viral vaccines, however, comes with specific challenges, particularly due to the demands of the cell substrates and viral production processes involved.
Several cell lines, including VERO, MDCK, MRC5, BHK, and CHO, are utilized in vaccine production. BHK-21 cells, in particular, are widely used for their effectiveness in vaccine production. Recent developments have shown that BHK-21 cells can be grown in microcarriers within single-use (SU) bioreactors, transitioning from conventional bioreactors. This study aims to scale up this process, highlighting the flexibility and efficiency of SU bioreactors for rapid scale-up without compromising product quality.
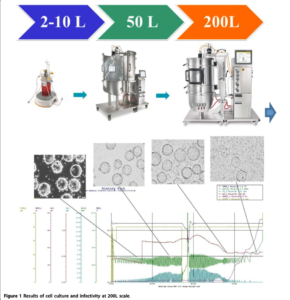
The study details the scaled-up production of a bovine viral vaccine, identifying optimal parameter values, testing intermediate bioreactor scales from 2 liters to 50 liters, and applying new process settings to a 200-liter industrial bioreactor. Ensuring homogeneous cell adhesion to microcarriers is crucial in microcarrier-based cell culture, making the evaluation of mixing and aeration performance vital for successful scale-up.
The primary goal was to evaluate the potential of SU bioreactors to produce antigens for animal vaccines as an alternative to roller bottles, maintaining product quality. Additionally, the study aimed to assess potential cost of goods (COG) reductions.
Key Findings and Conclusions
• Linear Scalability: The process demonstrated linear scalability from R&D to manufacturing
• Performance Parity: SU bioreactors provided similar growth and productivity as conventional bioreactors or roller bottles, with added flexibility.
• Operational Efficiency: SU bioreactors allowed for faster turnaround times between batches due to reduced cleaning and sterilization requirements.
• Containment Suitability: SU bioreactors are ideal for use in BSL-3 laboratories, where containment is crucial.
• Consistent Results Across Scales: Comparable results were observed across 2L, 10L, 50L, and 200L scales in terms of cell density, viability, productivity, and product quality.
• Effective Agitation and Speed Calculation: The bioreactor system ensured effective agitation and straightforward speed calculations during scale-up.
• Homogeneous Cell Growth: Equivalent cell growth profiles and cell population homogeneity on microcarriers were achieved, with good cell attachment and propagation.
The study confirms that SU bioreactors provide a viable process for producing bovine vaccines using attachment-dependent cells.
Future Prospects
A key future objective is to reduce COG by minimizing the number of roller bottles needed to inoculate the bioreactor and increasing virus yields, thereby enhancing cost-efficiency.
At Smart MCs, we are dedicated to advancing bioreactor technology to support high-quality vaccine production. Our commitment to innovation and scalability aims to meet the increasing demands of the biotech industry while ensuring the highest standards of product quality and operational efficiency.
For more information on our innovative solutions and to stay updated on the latest developments, visit our website or contact our team at Smart MCs.





Leave A Comment