Did you know that microcarriers are not only used for large-scale cell expansion, but also for therapeutic regeneration?
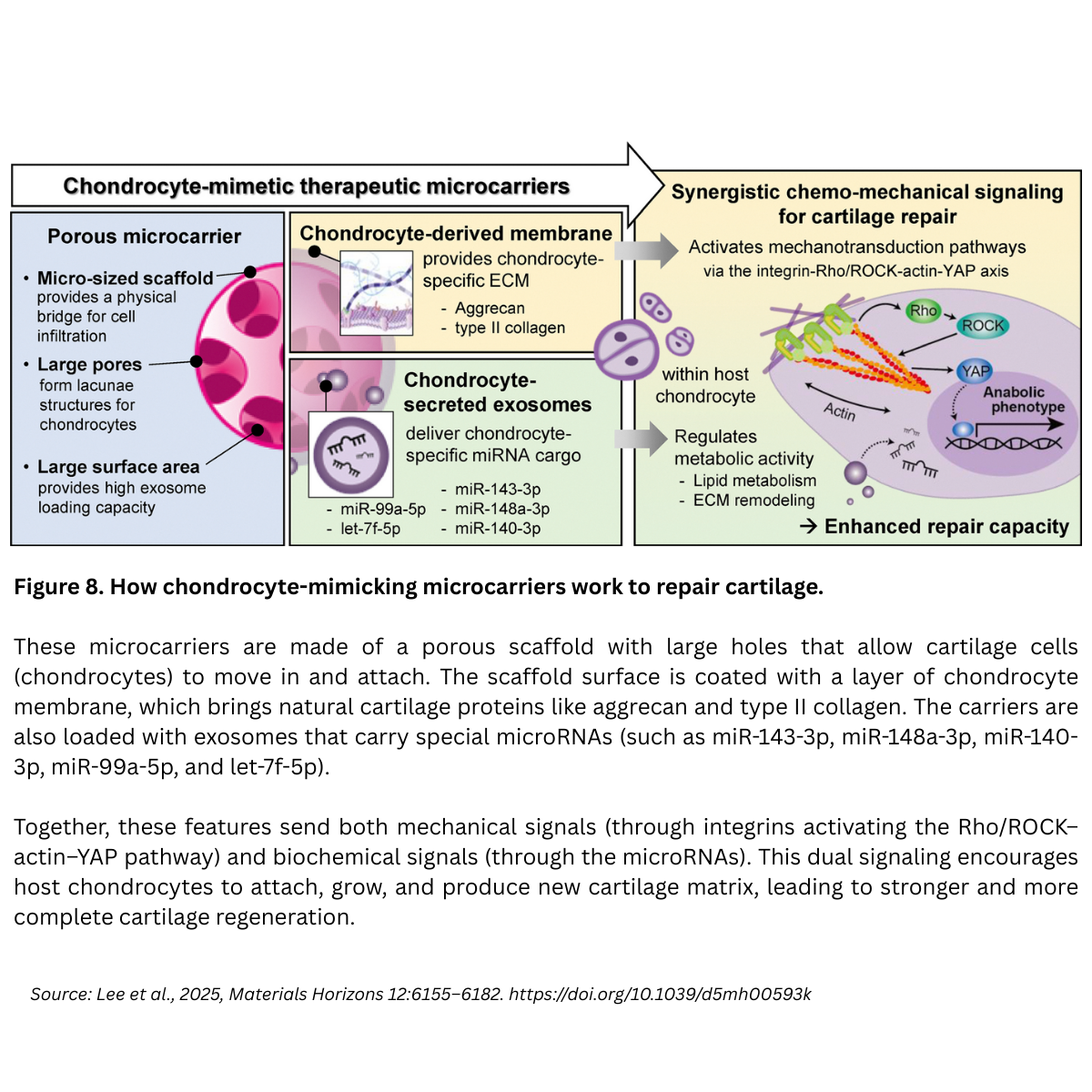
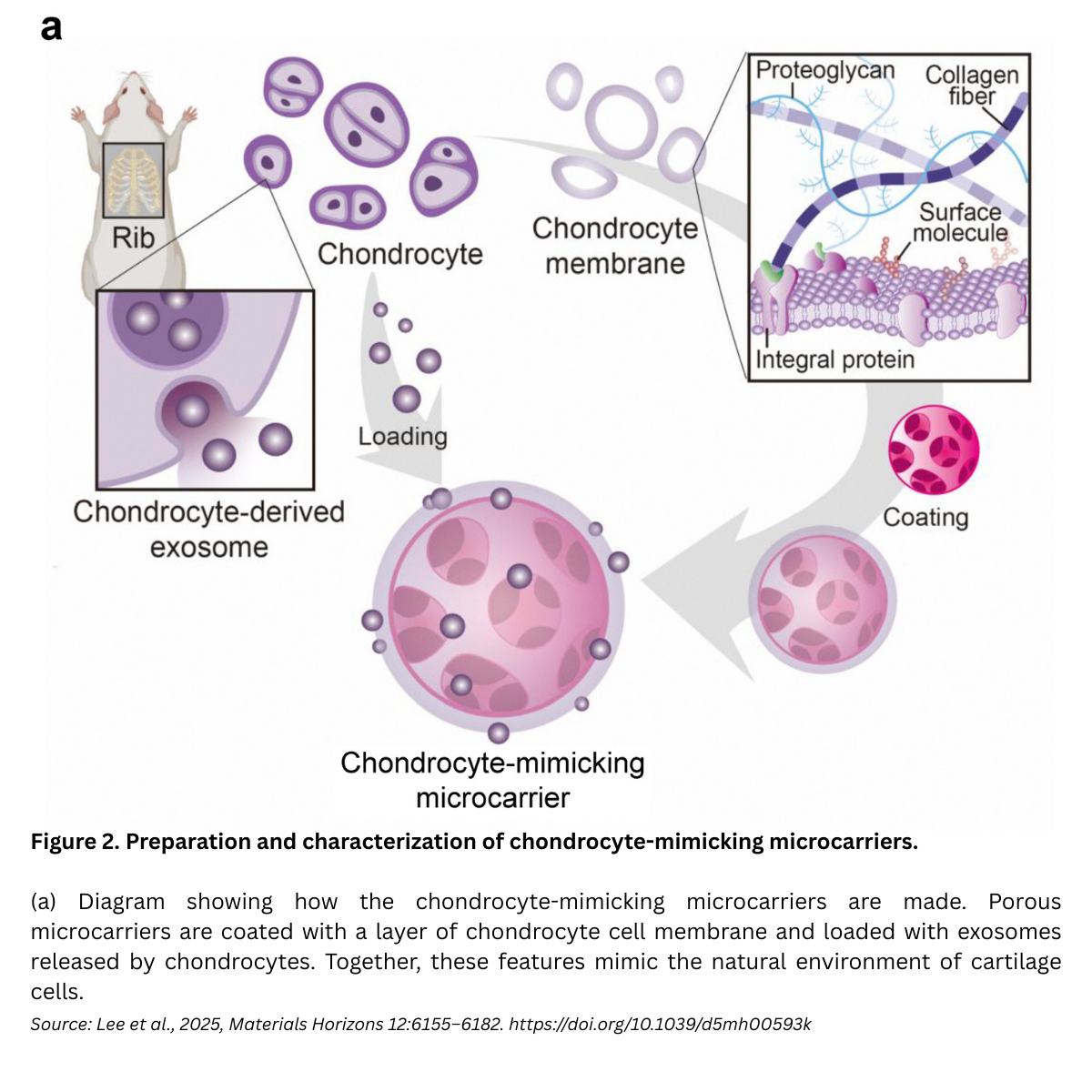
A recent communication in Materials Horizons by Na-Hyun Lee, Hye Sung Kim, Hae-Won Kim, and colleagues (Dankook University, South Korea), titled “Chondrocyte-mimetic therapeutic microcarriers for synergistic chemo-mechanical signaling in cartilage regeneration,” reports chondrocyte-mimetic therapeutic microcarriers that recapitulate the cartilage niche and coordinate repair through synergistic chemo-mechanical signaling. These porous microcarriers are cloaked with chondrocyte membranes and loaded with chondrocyte-secreted exosomes to drive host cell recruitment, proliferation, and maturation, offering a cell-free, clinically relevant strategy for cartilage regeneration.
Why microcarriers for cartilage repair?
Cartilage is avascular and aneural, which severely limits intrinsic healing. Conventional procedures (e.g., microfracture, mosaicplasty, autologous chondrocyte implantation) often struggle to restore native structure and function. In contrast, porous microcarriers can (i) deliver bioactive cargo, (ii) present a 3D matrix that permits oxygen and nutrient exchange, and (iii) be tuned in particle and pore size to emulate lacunae-like niches that regulate chondrocyte behavior. These attributes make microcarriers especially attractive for durable, integrative cartilage repair.
What the new study built: a chondrocyte-mimetic microcarrier
Design concept. The team engineered a porous polycaprolactone (PCL) microcarrier, then decorated it with (1) a chondrocyte-derived cell membrane (retaining cartilage-specific ECM components) and (2) chondrocyte exosomes (enriched in chondrogenic miRNAs). The goal: synchronize mechanical cues (via the membrane/ECM interface) with biochemical cues (via exosome delivery) to stimulate host chondrocytes at the defect site.
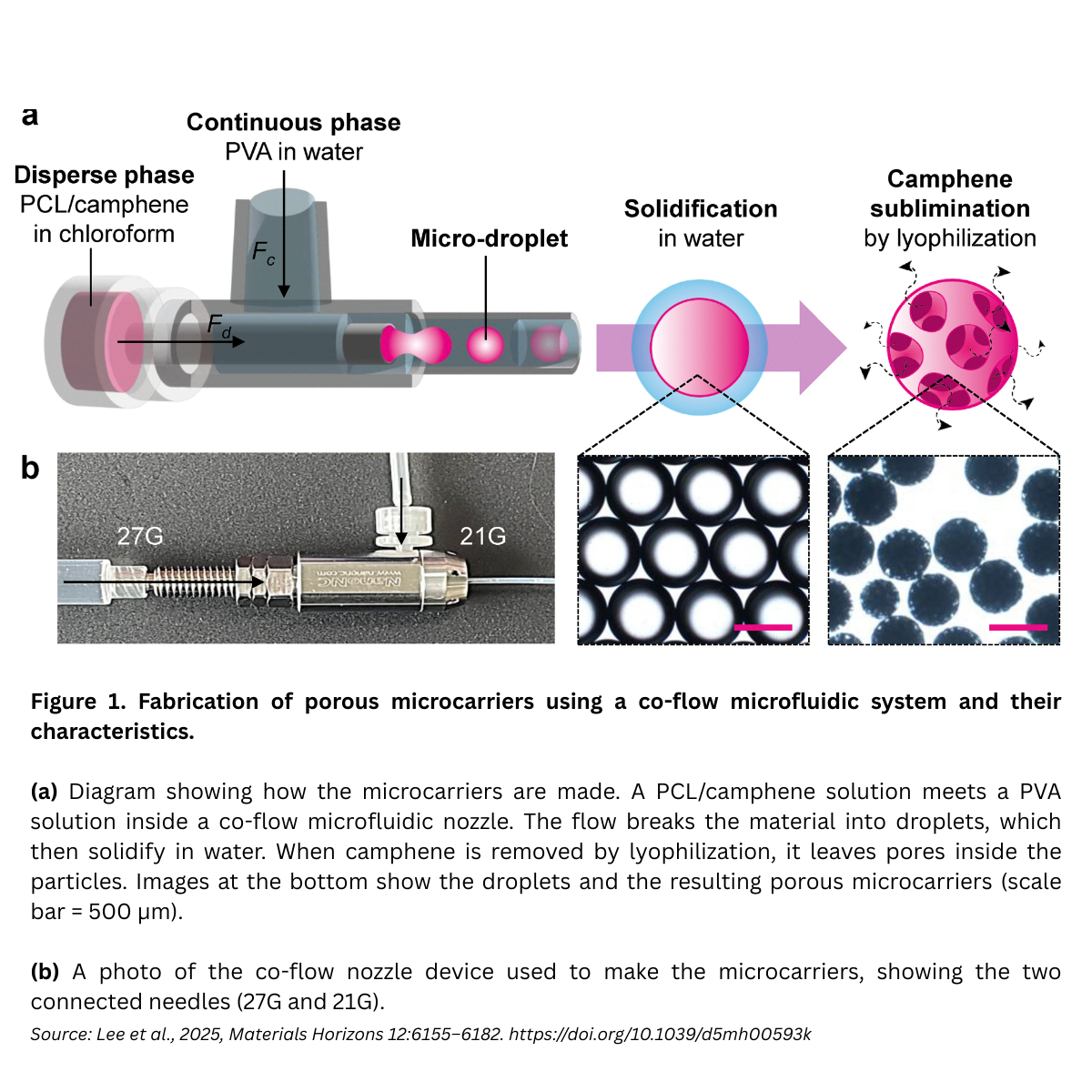
Fabrication & physical properties (co-flow microfluidics)
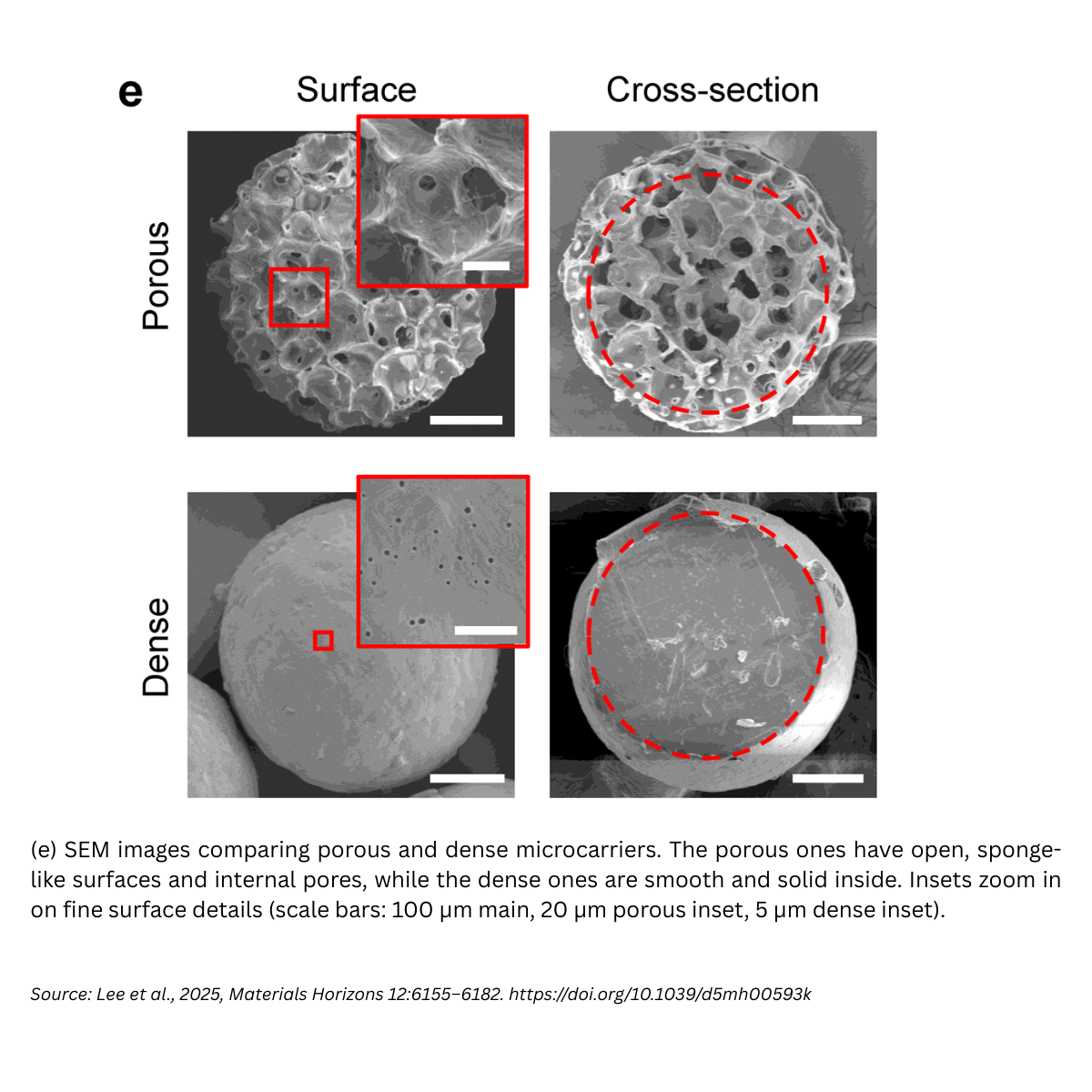
Porous PCL microcarriers were produced using a co-axial, co-flow microfluidic device. Under optimized conditions, 3% (w/w) PCL + 20% (w/w) camphene in the dispersed phase at 1 mL h⁻¹, with a PVA continuous phase at 10 mL h⁻¹, the carriers measured ~454.5 ± 5.4 µm with uniform pores (~59.6 ± 11.1 µm), and a remarkably tight size distribution (CV ≈ 1.2%). Compared to dense (non-porous) beads, the porous architecture lowered bulk modulus (~0.4 ± 0.1 MPa, close to native cartilage) and increased degradability.
Why this matters. Particle sizes in the ~300–500 µm range effectively pack irregular defects while remaining easy to handle; ~50–100 µm pores form lacunae-like niches that support chondrocyte anchorage, infiltration, and long-term function.
Membrane cloaking: making the surface “feel” like cartilage
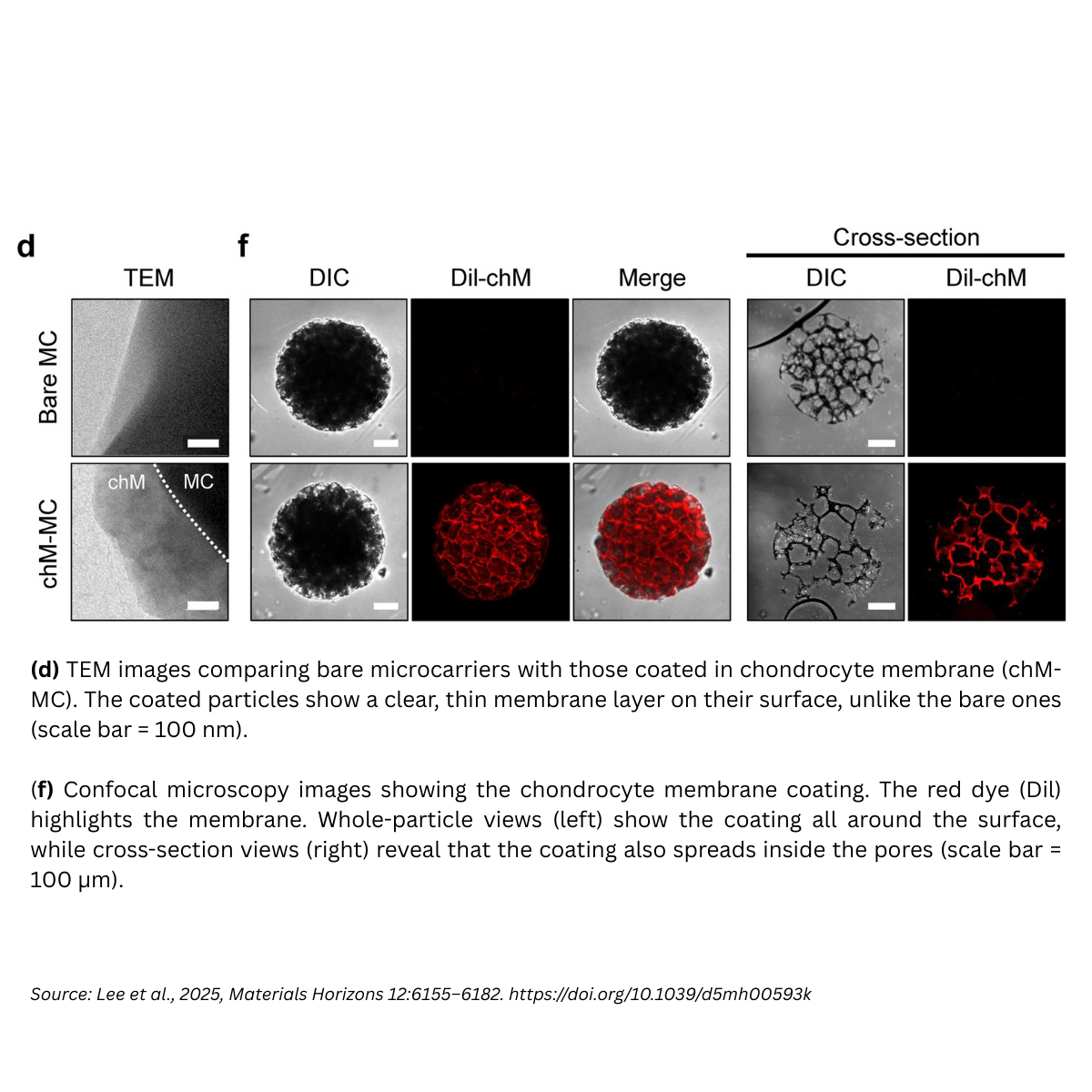
Coating the porous carriers with chondrocyte membranes created a thin (≈ 50–200 nm) uniform layer across surfaces and internal pores. This dramatically softened the surface from ~30 kPa (bare) to ~0.5 kPa (coated) closer to chondrocyte softness (0.5–1 kPa) and increased hydrophilicity. These surface changes act as bioactive cues, enhancing focal adhesion formation and cell–matrix signaling on the microcarrier.
Exosome cargo: cartilage-specific biochemical signals
The chondrocyte-derived exosomes (chExo) were ~116.9 ± 33.6 nm vesicles, positive for canonical exosome markers (TSG101, ALIX, CD63) and negative for organelle contaminants (Calnexin, GM130), confirming purity. When integrated with the membrane-coated microcarriers, they act as a reservoir for sustained, localized release of chondrogenic miRNAs (e.g., miR-140-3p, miR-143-3p), restoring cartilage homeostasis by modulating ECM remodeling and lipid metabolism pathways.
Mechanism: integrin → Rho/ROCK–actin → YAP (chemo-mechanical synergy)
The cell-membrane cloak recapitulates the native cell–matrix interface, engaging integrins and activating the Rho/ROCK–actin–YAP mechanotransduction axis. On these coated carriers, chondrocytes showed increased spreading, focal adhesion formation, and nuclear YAP localization events that elevated chondrogenic gene expression (Sox9, Col2a1, Acan). Inhibition studies (e.g., ROCK inhibitor Y-27632, YAP inhibitor verteporfin) suppressed nuclear YAP and downregulated chondrogenic programs, underscoring pathway causality.
Key takeaway. The platform coordinates mechanical (soft, ECM-bearing surfaces; lacunae-like pores) and biochemical (exosomal miRNAs) inputs to recruit, proliferate, and mature host chondrocytes, a dual mode that outperforms “mechanics-only” or “biochemistry-only” strategies.
In vitro and in vivo readouts
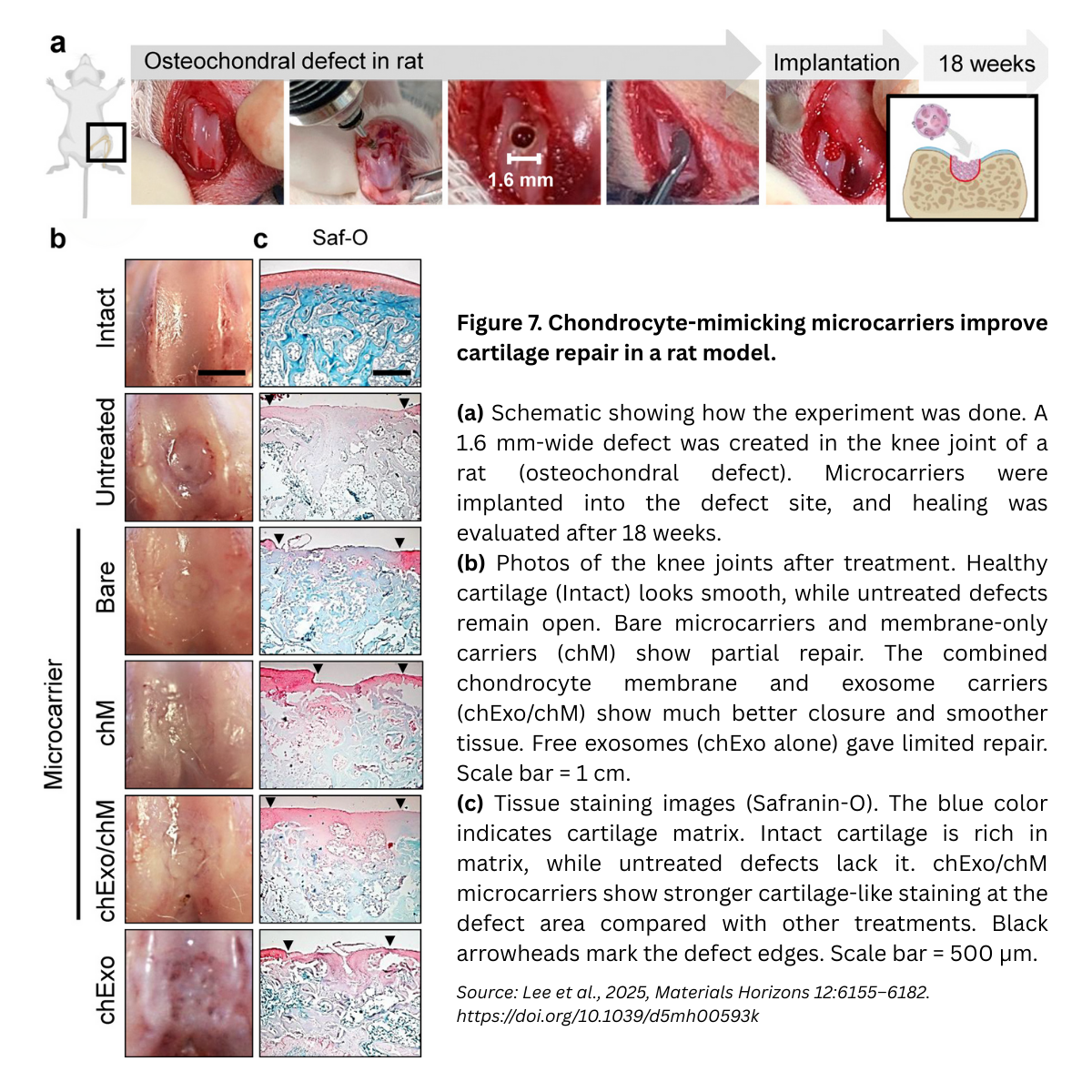
- In vitro maturation. On membrane-coated carriers, chondrocytes deposited robust ECM (Safranin-O, collagen II), consistent with anabolic signaling and YAP activation; quantitative image analyses and heatmaps confirmed higher chondrogenic gene expression than on uncoated carriers.
- In vivo (rat osteochondral defect model). The study evaluated microcarrier formulations in critical-size knee defects, followed by endpoint histology (H&E, Masson’s Trichrome, Saf-O/Fast Green, Collagen II IHC) and ICRS macroscopic scores. The membrane+exosome design supported integrative repair with improved cartilage-like matrix at harvest.
Together, the data support a cell-free, scalable approach that coordinates mechanotransduction and miRNA-guided remodeling to enhance cartilage repair.
A New Concept in Tissue Regeneration
What makes this research especially exciting is that it introduces a new concept in regenerative medicine: synergistic chemo-mechanical regulation using engineered microcarriers. Traditional approaches often focus on either biochemical cues (e.g., growth factors, exosomes) or biomechanical signals (e.g., scaffold stiffness, porosity). This study shows, for the first time, how combining both within a single microcarrier platform can activate mechanotransduction pathways (Rho/ROCK–actin–YAP) while simultaneously delivering exosomal miRNAs that drive extracellular matrix remodeling.
By synchronizing these two dimensions, the chondrocyte-mimetic carriers achieved coordinated host cell recruitment, proliferation, and maturation something neither a growth factor injection nor a static scaffold could achieve alone. This dual-mode synergy represents a fresh conceptual paradigm for cell-free therapies, minimizing the risks of cell transplantation and offering a scalable, off-the-shelf solution for complex tissue defects.
How Smart MCs Can Help You Apply These Insights
The Dankook University study highlights how carefully engineered microcarriers can combine biochemical and biomechanical cues to achieve remarkable therapeutic outcomes. While these findings come from academic research using custom experimental carriers, the principles translate directly into broader applications across regenerative medicine, cellular agriculture, and bioprocessing.
At Smart MCs, we build customisable microcarriers that allow you to tune size, surface charge, coatings, stiffness, shape/morphology, and matrix composition to suit your target cell type. Our platform includes xeno-free, dissolvable, and edible options for translational and food-grade applications, plus streamlined workflows to make seeding and harvesting easier and more reliable.
Conclusion
This research underscores that microcarriers are far more than just tools for scale-up, they can also serve as therapeutic platforms, orchestrating the complex signals needed for tissue repair. As these academic advances move toward clinical translation, the need for flexible, customisable microcarriers will only grow.
If you’re looking to explore microcarrier applications in your own work, talk to us about Smart MCs customisable microcarriers, built to match your cell type, workflow, and regulatory needs. Explore options or buy microcarriers tailored to your application today.
References
- Lee N-H., Zhou Z., Yoon J-Y., Mandakhbayar N., Li C.J., Lee J-H., Kim H-W., Kim H.S. Chondrocyte-mimetic therapeutic microcarriers for synergistic chemo-mechanical signaling in cartilage regeneration. Materials Horizons. 2025; 12: 6155–6182. doi: 10.1039/d5mh00593k.




Leave A Comment