In a paper published by Yang and colleagues, titled “Stem cell recruitment polypeptide hydrogel microcarriers with exosome delivery for osteoarthritis treatment” in the Journal of Nanobiotechnology (2024), the authors describe a hydrogel microcarrier system designed to improve exosome delivery and support cartilage repair. Their work demonstrates how microfluidic fabrication and biomaterial engineering can help overcome persistent limitations in exosome-based OA therapy.
Osteoarthritis is a degenerative joint condition characterised by the gradual breakdown of articular cartilage, subchondral bone changes, inflammation of the joint lining, and elevated oxidative stress. It affects more than 240 million people globally and often leads to chronic pain, reduced mobility, and long-term disability. Current treatments, such as NSAIDs, corticosteroid injections, hyaluronic acid injections, and surgery, primarily address symptoms rather than slow or reverse tissue degeneration.
Exosomes derived from mesenchymal stem cells (MSCs) have attracted interest because they can modulate inflammation, support cartilage matrix production, and influence local cell behaviour. However, when injected directly, they are rapidly cleared from the joint, limiting their therapeutic potential.The fast turnover of synovial fluid contributes to this rapid loss, reducing residence time and therapeutic benefit.
Hydrogel microcarriers produced using microfluidic technologies offer a way to protect these therapeutic components and release them more gradually within the joint.
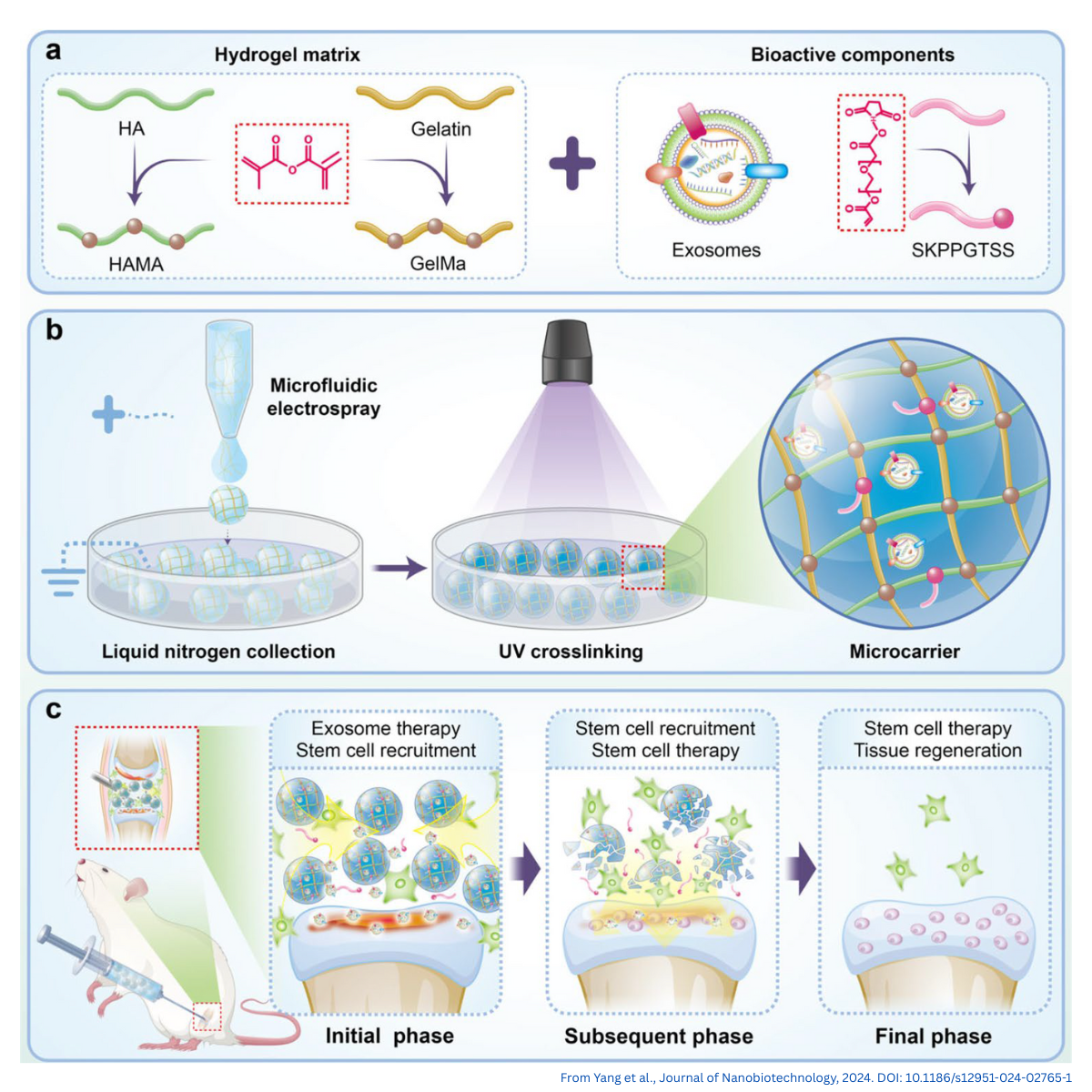
Figure 1. Overview of the HAMA/GelMA hydrogel microcarrier system for osteoarthritis research.
(a) Composition of the microcarriers, combining a HAMA/GelMA hydrogel matrix with two bioactive components: MSC-derived exosomes and the stem-cell–recruiting peptide SKPPGTSS. (b) Microfluidic electrospray is used to generate uniform droplets, which are collected in liquid nitrogen and UV-crosslinked to form porous, injectable microcarriers. (c) After intra-articular injection, the microcarriers provide an initial phase of exosome release and stem-cell recruitment, followed by enhanced endogenous stem-cell participation and tissue repair in later phases.
Why OA Research Benefits from Structured Delivery Systems
Standard OA treatments do little to alter the biological progression of cartilage degeneration. While MSC-derived exosomes offer promise, they face several key limitations within the joint environment:
- Fast clearance from the synovial cavity
- Limited retention at cartilage defect sites
- Short-lived therapeutic concentration
- Variability between injections
The synovial cavity is the fluid-filled space inside a synovial joint (such as the knee). It contains synovial fluid, which lubricates the joint and reduces friction during movement. Because the fluid is continuously circulated, nutrients and waste are exchanged rapidly, and therapeutic molecules injected into the synovial cavity are often cleared quickly. This natural turnover is one of the main reasons exosome injections have limited residence time.
These constraints make OA an ideal model for engineered delivery systems that can maintain bioactivity for extended periods, enabling more consistent therapeutic outcomes.
What Hydrogel Microcarriers Are
Hydrogel microcarriers are microscale, water-rich particles engineered to encapsulate biological payloads such as exosomes, peptides, cells, or growth factors. Their structure allows:
- Protection of sensitive biological cargo
- Tunable and sustained release
- A 3D microenvironment similar to extracellular matrix
- Injectability for minimally invasive delivery
In the referenced study, the microcarriers were composed of:
- GelMA (gelatin methacryloyl) – provides adhesion cues and structural flexibility
- HAMA (hyaluronic acid methacryloyl) – contributes lubricity, hydration, and biocompatibility
Both biomaterials are well-established in cartilage and joint repair applications due to their biocompatibility, hydration, and ECM-mimicking properties.
How These Microcarriers Are Made
The microcarriers were generated using a microfluidic electrospray process, which produces droplets with highly uniform size. A HAMA/GelMA pre-gel solution is pushed through a fine capillary under an electric field to form spherical droplets. These are collected in liquid nitrogen to stabilise their structure, then UV-crosslinked to create solid hydrogel particles.
Exosomes and the stem-cell–recruiting peptide SKPPGTSS are mixed into the pre-gel solution beforehand, enabling gentle encapsulation without damaging the biological components. The peptide is first acrylated so it can be covalently crosslinked into the hydrogel during UV exposure, improving retention and preventing premature release
Microfluidic Hydrogel Microcarriers as a Delivery Platform
The fabrication method results in particles with:
- Uniform, injectable morphology
- Controlled size distribution for predictable degradation
- Porous internal architecture supporting sustained exosome release
These characteristics make the microcarriers suitable for controlled delivery applications in OA research.
Integrated Stem-Cell Recruitment Capability
The SKPPGTSS peptide was incorporated directly into the hydrogel network during UV crosslinking, resulting in:
- Stable retention within the microcarrier
- Reduced premature peptide release
- Active recruitment of endogenous stem cells toward cartilage defects
This provides a complementary mechanism alongside exosome delivery.
Sustained Exosome Release
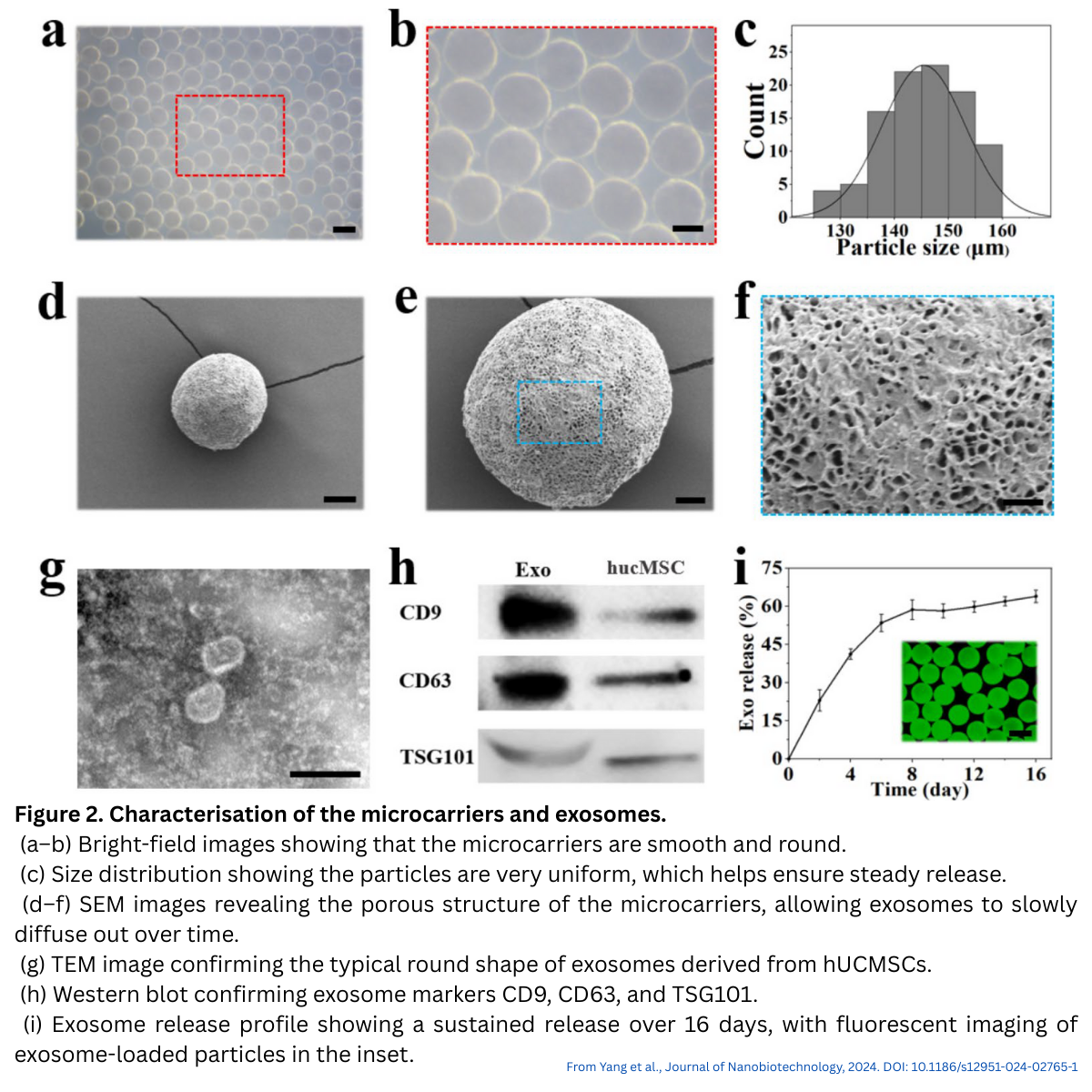
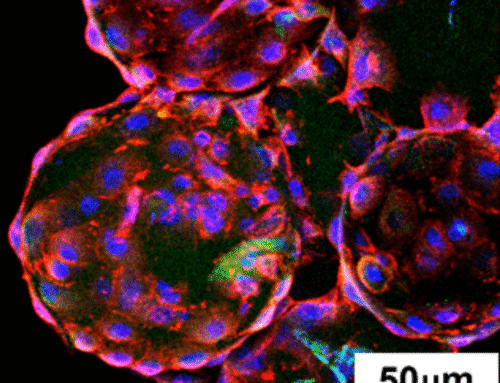
Exosomes derived from human umbilical cord MSCs were uniformly encapsulated throughout the microcarriers. Release studies showed:
- Approximately 60% release in the first 8 days
- Continued release with hydrogel degradation
- Preservation of exosome morphology and markers (CD9, CD63, TSG101)
This sustained profile is a major advantage compared with single-dose intra-articular injections.
Treatment Groups Used in the Study
To evaluate the microcarriers, the researchers compared several experimental groups:
- Control – untreated cells
- H₂O₂ – cells exposed to oxidative stress (OA-like damage)
- Par – blank microcarriers (no peptide, no exosomes)
- Par@Pep – microcarriers loaded with the SKPPGTSS peptide
- Par@Exo – microcarriers loaded with MSC-derived exosomes
- Par@Pep&Exo – microcarriers containing both peptide and exosomes (full formulation)
This grouping allowed the researchers to determine how each component contributed to cell protection and cartilage repair.
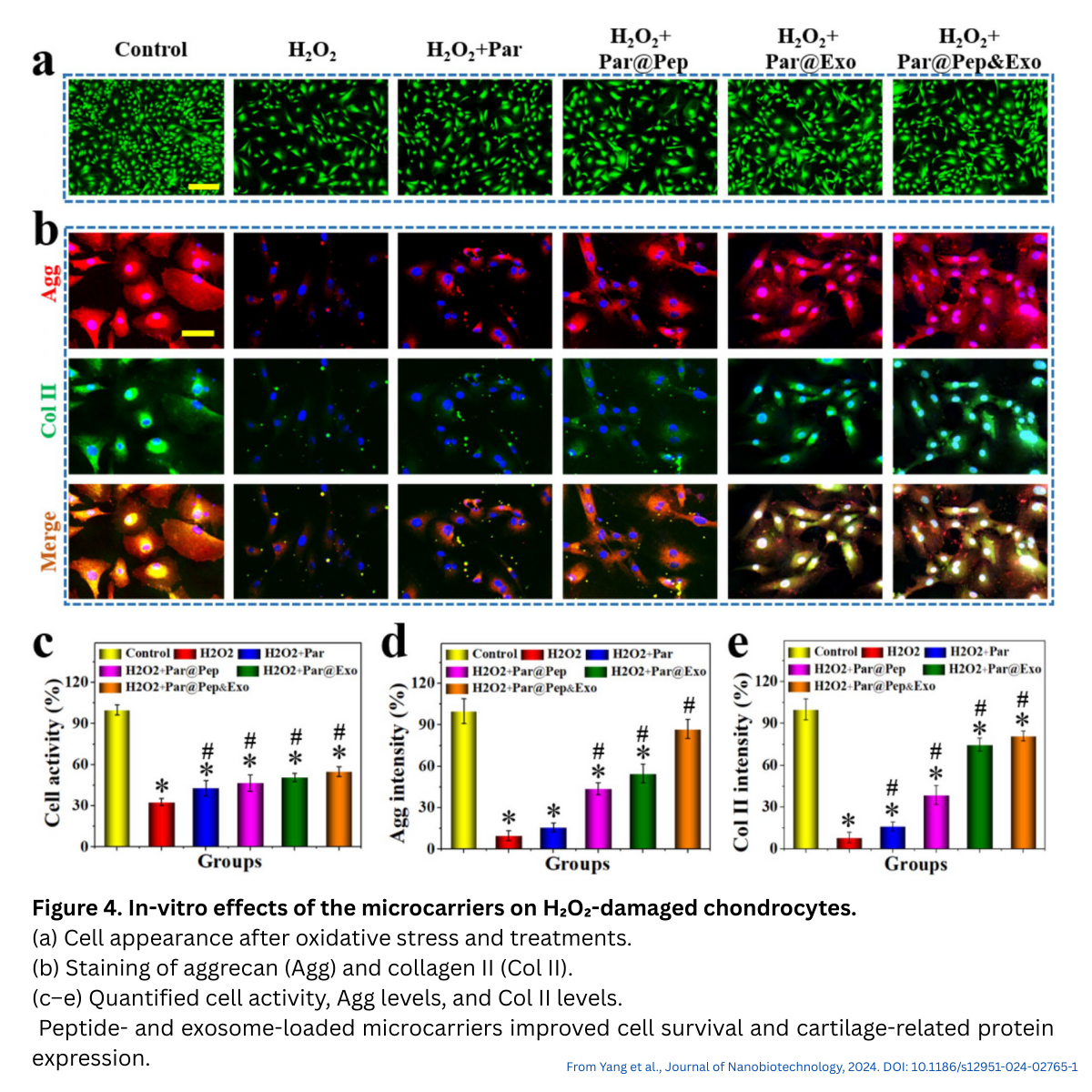
Key Observations From In Vitro Studies
Several in vitro assessments were performed:
- Stem cell recruitment: Stem cell recruitment increased in groups containing peptide (Par@Pep, Par@Pep&Exo) and to a lesser extent in Par@Exo.
- Chondrocyte protection: Under oxidative stress (H₂O₂), microcarriers improved viability and maintained collagen II and aggrecan expression.
- Chondrogenic differentiation: Exosome-loaded carriers enhanced GAG deposition, with Par@Pep&Exo showing the strongest effect.
These findings indicate support for both early protection and later-stage regenerative processes.
In Vivo Findings in an OA Model
In a rat OA model, the microcarriers were injected bi-weekly over eight weeks. The study reported:
- Reduced cartilage erosion and surface irregularities
- Higher GAG deposition in Safranin O–Fast Green staining
- Lower OARSI scores compared with untreated controls
- Higher collagen II and aggrecan expression
The combined Par@Pep&Exo formulation consistently produced the strongest therapeutic benefit.
Implications for Biomaterials and Delivery System Design
Key considerations illustrated by this work include:
- Microfluidic fabrication enables reproducible particle generation.
- HAMA/GelMA hydrogels provide biocompatibility and lubrication suited to joint environments.
- Recruitment peptides allow microcarriers to play an active role in tissue repair.
- Encapsulation improves exosome stability and prolongs their therapeutic window.
This approach may inform future biomaterial-assisted strategies for OA and related musculoskeletal disorders.
How This Relates to Smart MCS Technologies
Smart MCS offers materials and tools that align closely with this research, including:
- Photocrosslinkable hydrogels for encapsulation
- Microcarriers for culture and delivery
- Microfluidic chips for consistent particle formation
- Custom biomaterial formulations for controlled release
These capabilities support OA research, exosome delivery development, and broader tissue engineering applications.
Disclaimer
Smart MCs is not affiliated with the authors of the referenced study. The researchers did not use Smart MCs products in their experiments. This article summarises published findings for educational and informational purposes only.
Reference:
Yang, L., Li, W., Zhao, Y., Wang, Y., & Shang, L. (2024). Stem cell recruitment polypeptide hydrogel microcarriers with exosome delivery for osteoarthritis treatment. Journal of Nanobiotechnology, 22(1). https://doi.org/10.1186/s12951-024-02765-1



Leave A Comment