A pivotal shift toward human-relevant science, regulatory alignment, and the adoption of New Approach Methodologies (NAMs)

In July 2025, the U.S. Food and Drug Administration (FDA) and the National Institutes of Health (NIH) jointly hosted the FDA-NIH Workshop: Reducing Animal Testing, a pivotal event in biomedical research policy. With broad participation from regulatory bodies, industry scientists, academic researchers, and international partners, the workshop outlined a clear and coordinated pathway to reduce and eventually replace animal testing with modern, human-relevant methodologies.
The workshop marked a shift from discussion to execution. Following recent legislative and regulatory changes including the FDA Modernization Act 2.0 and the release of the FDA’s “Roadmap to Reducing Animal Testing in Preclinical Safety Studies,” the focus is now on accelerating the development, validation, and adoption of scientifically sound alternatives.
Inside the Workshop: Key Highlights
Held as a hybrid event on 7 July 2025, the FDA-NIH Workshop: Reducing Animal Testing brought together regulators, scientists, and policy experts to outline practical steps for moving beyond animal-based research. A central theme was strategic implementation, starting with the FDA’s roadmap to phase out animal testing for monoclonal antibody safety studies. The event also focused on the validation of New Approach Methodologies (NAMs), with agencies like ICCVAM playing a key role in standardising protocols. Efforts to align regulatory review processes across the FDA and NIH were discussed in depth, alongside a major policy update from NIH: animal-only studies will no longer be eligible for funding under new grant programs. Importantly, the workshop stressed the need for public engagement and transparency, acknowledging that trust and education are essential for widespread adoption of non-animal methods.
Why Animal Testing Is Being Phased Out
1. Low Predictive Power for Humans
Animal studies often fail to translate into accurate human outcomes. Despite decades of use, over 90% of drugs that pass animal trials fail in human clinical trials, either due to unexpected toxicity or lack of effectiveness. Differences in metabolism, immune function, and organ biology between animals and humans limit the reliability of traditional animal models.
2. Scientific Inefficiency
Animal-based research can be time-consuming, costly, and highly variable. For example, safety testing for monoclonal antibodies may require hundreds of animals and cost tens of thousands of dollars per compound. Such inefficiencies hinder innovation and delay the delivery of life-saving treatments.
3. Ethical and Public Pressure
There is growing public demand to reduce the use of animals in science. Modern ethics, coupled with the availability of advanced alternatives, have intensified scrutiny on animal-based practices. In many countries, including the U.S., UK, and EU member states, regulators and funding bodies are under increasing pressure to support more humane approaches.
What Are New Approach Methodologies (NAMs)?
New Approach Methodologies (NAMs) are a collection of innovative tools and techniques that offer scientifically validated alternatives to traditional animal testing. NAMs include:
- Organ-on-a-Chip systems: Microengineered devices that simulate human organ functions, such as liver toxicity or lung inflammation.
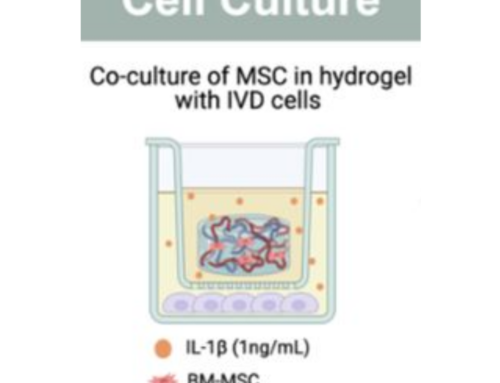
- 3D Cell Cultures and Organoids: Miniature tissues grown from human cells that replicate the structure and behaviour of real organs.
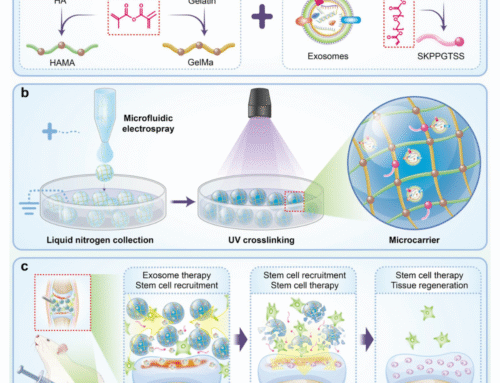
- Microfluidic Platforms: Systems that mimic blood flow, nutrient delivery, and cellular interactions in real time.
- Computational and AI Models: In silico simulations that predict pharmacokinetics, toxicity, and drug–target interactions based on human data.
- High-Throughput Screening with Human Cells: Rapid testing methods using diverse human-derived cells to evaluate drug effects across populations.
NAMs are not only more humane, they’re also often faster, cheaper, and more predictive of human outcomes.
The FDA Roadmap in Detail
In April 2025, the FDA released its official “Roadmap to Reducing Animal Testing in Preclinical Safety Studies,” a strategic document outlining short- and long-term goals:
- Initial Focus: Replace animal testing requirements for monoclonal antibodies using validated NAMs.
- Pathway Expansion: Apply validated methods to broader drug classes (e.g., small molecules, gene therapies).
- Infrastructure Development: Improve data sharing, standards for validation, and lab access to qualified non-animal tools.
- Global Alignment: Work with international regulators to harmonise guidelines and accept NAM data for global submissions.
The roadmap emphasises that reducing animal testing is not only ethically necessary, but also essential for improving scientific integrity and public health outcomes.
Global Implications
Although this workshop was U.S.-led, its outcomes are likely to have a significant impact on regulatory practices worldwide. The European Medicines Agency (EMA), the UK’s DEFRA, and Health Canada are among the agencies already exploring the integration of NAMs into their approval frameworks. The OECD is also working on standardisation of alternative test guidelines.
This global alignment means that innovations validated under FDA and NIH frameworks could soon become the international standard accelerating drug development across borders.
Challenges Ahead
The workshop also acknowledged key challenges to widespread NAM adoption:
- Validation and Acceptance: NAMs must undergo rigorous validation to meet regulatory standards.
- Education and Training: Researchers and reviewers need upskilling to interpret NAM data effectively.
- Infrastructure Gaps: Some labs may lack access to NAM platforms or the expertise to implement them at scale.
- Cultural Change: Shifting decades-old habits and preferences for animal models requires strong leadership and long-term commitment.
Despite these hurdles, the consensus at the workshop was clear: the scientific, ethical, and regulatory case for reducing animal testing is stronger than ever.
Conclusion
The FDA-NIH Workshop: Reducing Animal Testing represents a transformative moment in biomedical science. It reflects a coordinated, strategic move away from reliance on animal data and toward a future defined by accuracy, efficiency, and compassion.
With government leadership, scientific innovation, and public support now aligned, the transition to New Approach Methodologies is not only possible… it is inevitable.
Smart MCs’ Role in the Transition Beyond Animal Testing
Smart MCs supports the global transition away from animal testing by delivering technologies that advance human-relevant research. Our hydrogels, organ-on-a-chip systems, and microfluidic platforms are built for predictive, ethical preclinical studies, while our microcarriers enable the scalable growth of 3D cell cultures used in New Approach Methodologies (NAMs). To see how our products align with the FDA and NIH vision for modern toxicology and drug development, get in touch or explore our full range here.



Leave A Comment