
In the world of vaccine manufacturing, achieving the highest level of efficiency and consistent output is imperative. Seen especially when addressing the urgent demand for widespread immunisation, combating infectious diseases, and responding to global health crises. An article published by Cell Culture Dish, outlined the recent innovations in accelerating vaccine production from Vero cells through utilisation of microcarriers among other key methods. See Original Article.
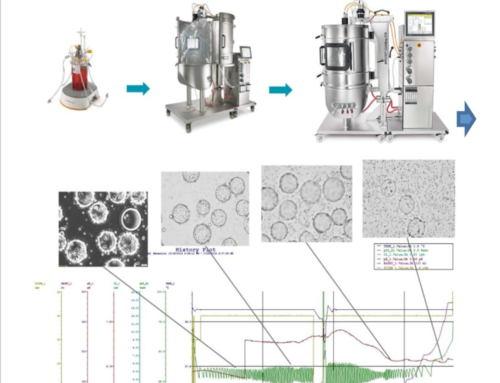
The increasing demand for viral disease vaccines has led to the development of advanced production techniques, including perfusion cell culture. This method utilises a cell retention device and continuous media exchange to maintain high cell densities and viabilities over extended periods. The article discusses strategies and technologies for expediting vaccine development, especially during the COVID-19 pandemic. It highlights the use of upstream bioprocessing expertise and single-use technologies to accelerate scale-up. It explores Vero cell-based vaccine production and optimisation strategies using suspension bioreactor cultures and serum-free media. The application notes cover cell culture and perfusion culture using microcarrier spin filters. High-density Vero cell perfusion culture in vessels is showcased for increased cell densities and virus yield.
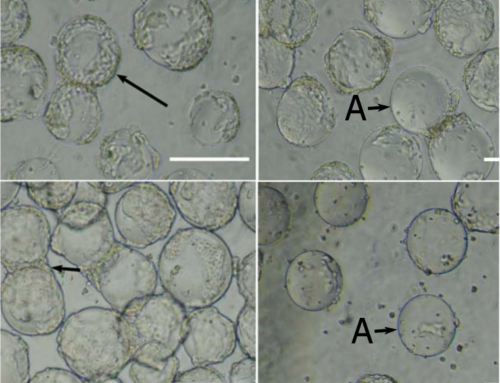
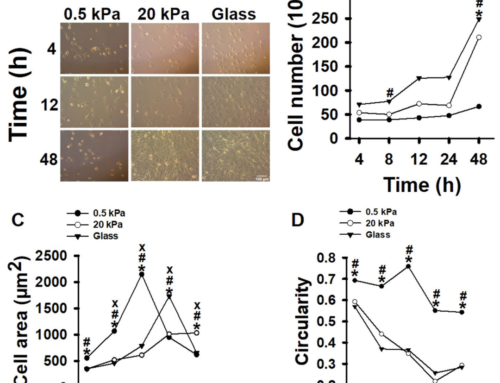
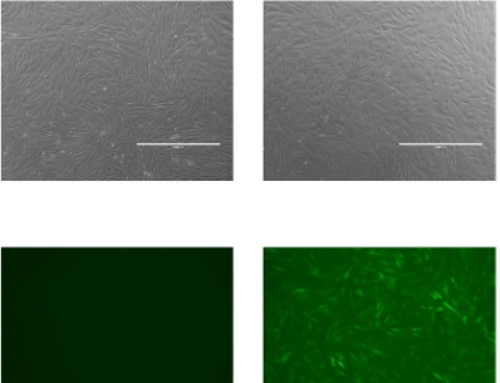
In the study, microcarriers were utilised in both bioreactor-based therapy and single use vessels. The results were then recorded. The study explores the use of sophisticated production techniques like perfusion cell culture, which maintains high cell densities and viabilities over extended periods. The use of a microcarrier spin filter as a cell retention device in conjunction with Bioreactor and Cell-Lift Impeller system demonstrates effectiveness in achieving high-density Vero cell cultures. The study concluded a Vero cell density of 8.0 × 106 cells/mL within a mere 9-day period at a modest microcarrier loading density of 10 g/L. This achievement is significant as it provides sufficient cell density to inoculate a 40L 510 packed-bed bioreactor intended for vaccine production, assuming a cell recovery rate exceeding 50%. Thus, the data showcases the immense potential of the platform for scaling up attachment cell-based vaccine production. These findings indicate the potential for scalable production of attachment cell-based vaccines using microcarrier technologies.
Within the production of vaccines and similar therapeutics, microcarriers have shown to ensure significant benefit when scaling up production. To learn more about our microcarrier products, visit our Knowledge Centre. To get in touch with our experts, email us: info@smartmcs.com.au
Original Article: Cell Culture Dish (Nov. 2022) Bioprocessing Solutions to Accelerate Mammalian Cell-Based Vaccine Development.

Leave A Comment