Recent research spearheaded by Stanford scholars has shed light on the pioneering use of induced pluripotent stem cells (iPSCs) in cardiac tissue engineering (Link to Source Article). This innovative approach not only promises to revolutionize our understanding of cardiovascular diseases but also paves the way for new therapeutic strategies. Here’s what you need to know about the groundbreaking work in iPSCs and heart reconstruction.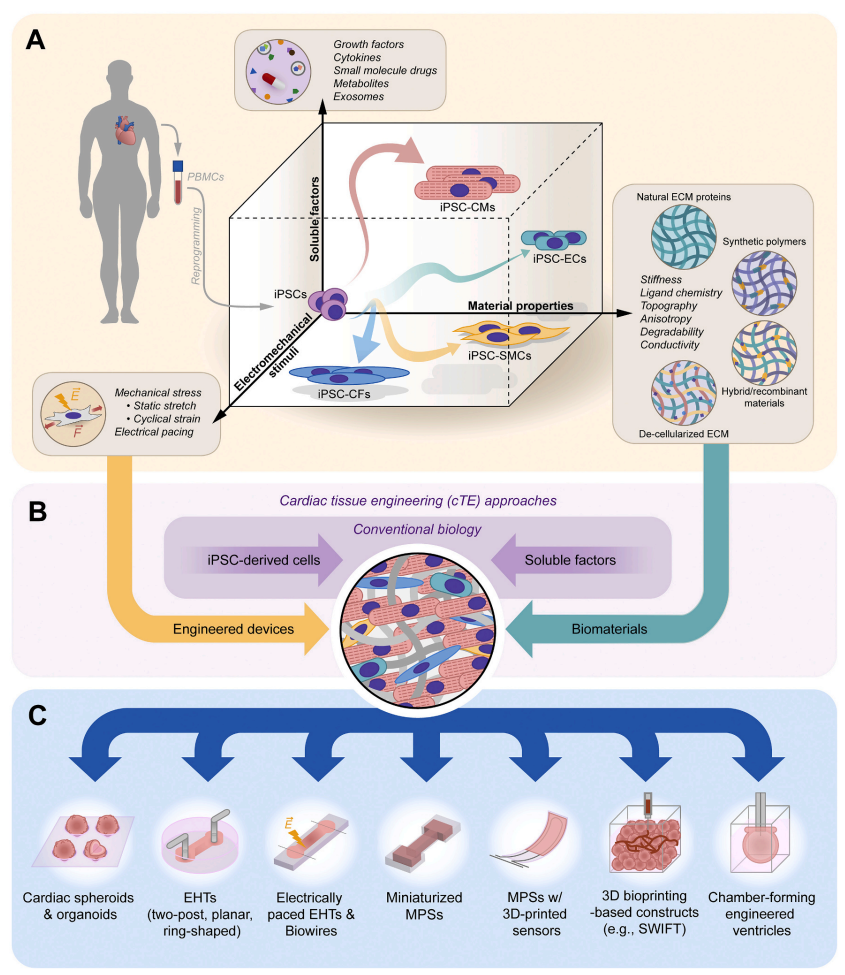
What are iPSCs?
Induced pluripotent stem cells, or iPSCs, are a type of stem cell that can be generated directly from adult cells. The iPSC technology allows for the development of an unlimited source of any type of human cell needed for therapeutic purposes. By reprogramming adult cells to an embryonic stem cell-like state, researchers can cultivate cells that are capable of producing any cell type in the human body, which are pivotal in the field of regenerative medicine.
Importance of iPSCs in Heart Reconstruction
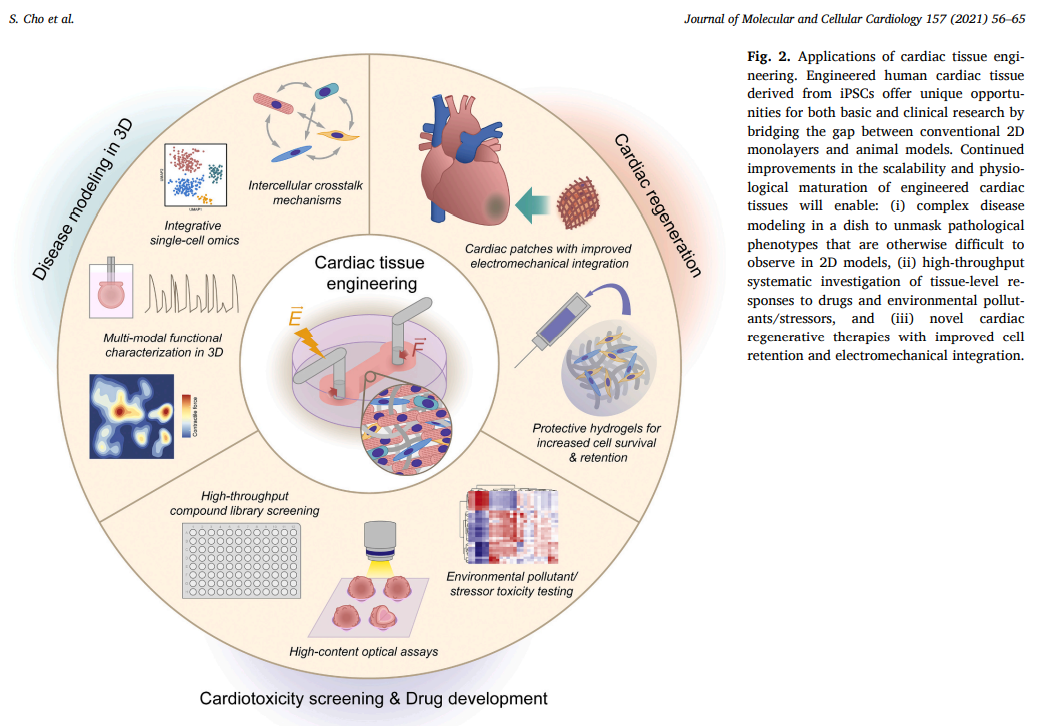
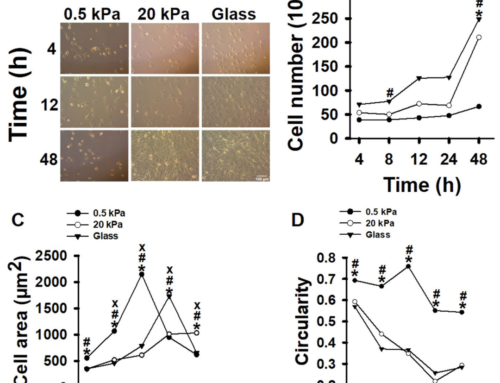
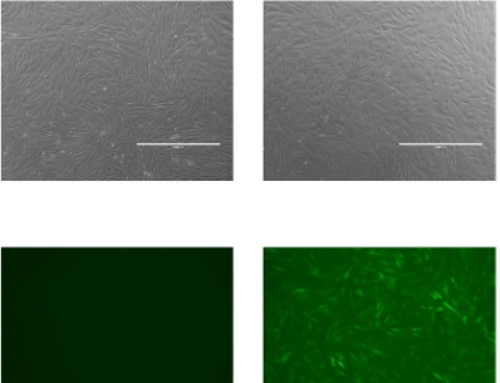
iPSCs are particularly significant for reconstructing the heart as they can be transformed into cardiac muscle cells, or cardiomyocytes, which integrate into existing heart tissue. These cells are invaluable for studying cardiovascular disease mechanisms, observing drug responses, and exploring developmental processes in human three-dimensional tissue models. iPSC-derived cardiac spheroids, organoids, and heart-on-a-chip models offer a spectrum of tools that mimic heart pathology and treatment in a controlled environment, enhancing our ability to simulate and study heart functions.
The Role of 3D Hydrogel Modeling
3D hydrogel modeling stands as a cornerstone technology in this domain. Hydrogels, which are highly hydrated networks of polymer chains that are biocompatible and closely mimic the natural environment of human tissues, provide a scaffolding in which iPSCs can grow and mature into functional cardiac tissues. These structures support the formation of cells with the right mechanical properties and biochemical interactions needed to simulate real human tissues.
Smart MCs and Hydrogel Solutions
An important aspect of successful 3D cell culture is the quality of hydrogels used. Smart MCs excels in this arena, offering a range of top-quality hydrogels such as GelMA, AlgMA, SilkMA, and HAMA. These products are specifically designed to meet the rigorous demands of iPSCs culture, ensuring that researchers have the most reliable and effective tools at their disposal. For more detailed information on these innovative products, be sure to visit our product page.
Challenges and Future Directions
Despite the promising advancements, several challenges remain in the cardiac tissue engineering (cTE) field. Issues such as inadequate vascularization, structural and functional immaturity of engineered tissues compared to native myocardium, and the limited proliferative capacity of iPSC-derived cardiomyocytes highlight the need for ongoing innovation and improvement. New strategies for enhancing vascularization, such as 3D bioprinting of vasculature and microfluidic systems, are under development to overcome these obstacles.
Conclusion
The field of cTE has evolved significantly over the past two decades, driven by advancements in iPSC technology and 3D modeling. Looking ahead, continuous improvements in tissue design and integration with other biotechnologies are expected to broaden the possibilities of disease modeling and regenerative cardiology. As these technologies converge with next-generation analytical tools, they hold the potential to transform cardiovascular research and treatment, heralding a new era of data-driven precision medicine.
For anyone involved in cardiovascular research or tissue engineering, the evolving landscape of iPSC application offers exciting opportunities and promises substantial impacts on healthcare outcomes in the foreseeable future.

Leave A Comment