In the realm of biotechnology, the quest for more efficient methods to cultivate cells for vaccine production is ongoing. A recent paper titled “Seed Train Optimization in Microcarrier-Based Cell Culture Post In Situ Cell Detachment through Scale-Down Hybrid Modeling” has offered a promising breakthrough that could significantly enhance the scalability and efficiency of viral vaccine manufacturing.
Overview of the Study
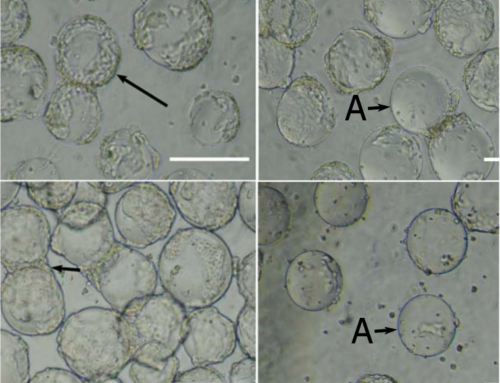
The research focuses on optimizing the cultivation process of anchorage-dependent cells, such as the MA 104 cell line, which are crucial for antigen production in vaccine manufacturing. Traditionally, these cells are propagated in static cell cultures before being seeded into production bioreactors with microcarriers (MCs). This study challenges the conventional methodology by demonstrating an effective strategy for serial subculturing on MCs with in situ cell detachment, all within closed culture units. Remarkably, this approach allows the MA 10^4 cell line to be subcultured at least five times on Cytodex 1 MCs without separating cells from MCs after cell harvest.
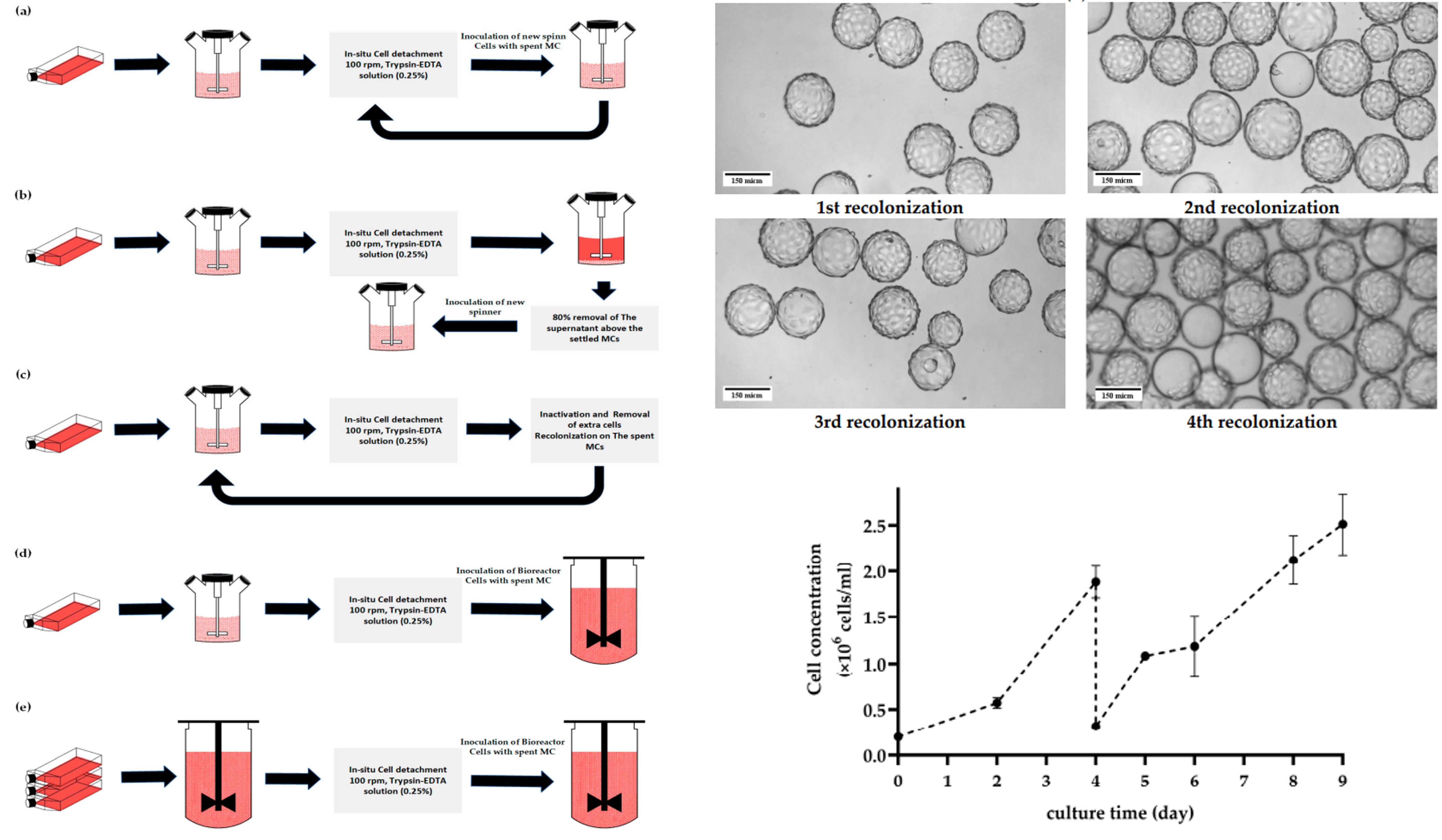
Key Findings and Innovations
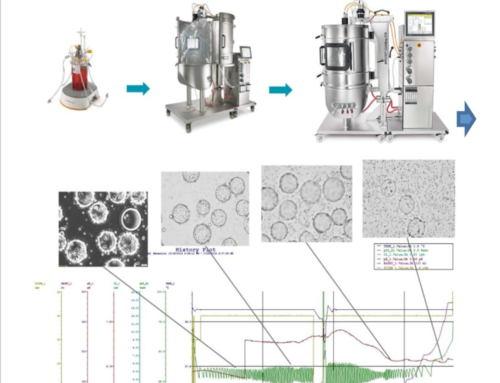
The paper highlights the success of using a scaled-down model to study process parameters affecting cell growth post in situ cell detachment. By employing an augmented Design of Experiments (DoE) alongside hybrid modeling, the researchers rapidly screened the design space for critical process parameters (CPPs), optimizing conditions to achieve optimal cell growth. These optimized conditions included an inoculation density of more than 16 cells per bead, Cytodex 1 concentration of 3.5–4.5 g/L, and controlled agitation speeds. Under these conditions, the study achieved a cell density of 2.6 ± 0.5 × 10^6 cells/mL after five days.
The study’s methodology presents a robust and efficient approach for seed training in stirred tank reactors. This is particularly beneficial for the production of viral vaccines, as it provides a reliable path for attaining the necessary cell volumes for antigen manufacturing. The research underscores the importance of optimized cell detachment methods and the novel aspect of repetitive recolonization of spent MCs, showcasing the MA 104 cells’ ability to resettle on the spent Cytodex 1 without additional cleansing steps.
Conclusions and Implications for the Industry
The research provides valuable insights into the MA 104 cell line’s behavior during MC cultivation, offering practical recommendations for implementing this methodology in scalable vaccine manufacturing. The study’s findings emphasize the potential of this innovative approach for seed training and cell propagation, marking a significant step forward in the quest for more efficient and scalable methods in vaccine production.
This groundbreaking research not only advances our understanding of cell behavior in microcarrier-based cultures but also lays down a practical framework for scaling up vaccine manufacturing processes, potentially transforming the way vaccines are produced on a global scale.
Source: https://doi.org/10.3390/bioengineering11030268

Leave A Comment